THE ROLE OF STRUCTURAL DIFFERENCES OF
FLAVANOLIGNAN SILYBIN STEREOIZOMERS IN BINDING TO HEPATOCYTES
J.
Šebestian1,2, Š.B. Šebestianová3, Z. Švagera4
and A. Jegorov5
1 Institute of Physical Biology, Univ. South
Bohemia, Nový Zámek 136, CZ-373 33 Nové Hrady, CZECH REPUBLIC
2 Dept. Plant Physiology, Fac. Biol., Univ.
South Bohemia, Branišovská 31, CZ-370 05 České Budějovice, CZECH REPUBLIC
3 Dept. Genetics, Fac. Biol., Univ. South
Bohemia, Branišovská 31, CZ-370 05 České Budějovice, CZECH REPUBLIC
4 Institute of Medical Chemistry and
Biochemistry, Fac. Med., Palacký Univ., Hněvotínská 3, CZ-775 15 Olomouc, CZECH
REPUBLIC
5 IVAX CR, Research Unit, Branišovská 31, CZ-370
05 České Budějovice, Czech Republic
Hepatoprotective effects of Milk Thistle (Silybum
marianum) have been known since ancient Greece and Roma very well.
Flavanolignans (called silymarine) extracted from Milk Thistle seeds were shown
to help against hepatotoxic effects of many natural toxins (i.e. alga toxin
microcystine, mushroom toxins amanitin and phaloidin, fungal toxins
cyclosporines, etc.). The main active substance of silymarin is silybin.
Recent studies revealed that many
transport and metabolic processes in the cell are stereospecific. Silybin
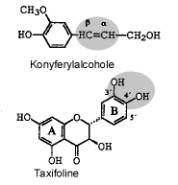
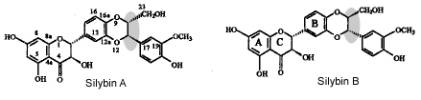
occurs in two stereoisomers (A and B) that differ in the bound between
konyferyl and taxifolin (Fig. 1). We developed a new method for preparation and
purification of these silybin stereoisomers and for their specific labelling by
radioactivity (3H, 125I) at positions 6 and 8. Transport of four stereoisomers
was studied. The best affinity of transport systems were found for 6-[125I]silybinA,
which is taken 100 times better than the other silybin stereoisomers.
Figure 1
This work was supported by grants GACR 204/98/P129, MSMT
CEZ:J06/98:123100001 and GACR 521/99/D098.
Jiri
Sebestian
Tel.: +420
38 7772349
Fax: +420
38 5310366
E-mail:
sebest@jcu.cz