Electron microscopy and single particle analysis of Photosystem II from red alga Porphyridium cruentum.
Ladislav Bumba1,2, Helena Havelková-Doušová3, Michal Hušák4 and František Vácha2,4
1Faculty. of Biological Sciences, University
of South Bohemia, Branišovská 31, 37005 Č. Budějovice, Czech republic
2Institute of Plant and Molecular Biology, Czech Academy of Sciences, Branišovská 31, 37005 Č.Budějovice, Czech republic
3 Institute of Microbiology, Division
of Autotrophic Microorganism, Czech Academy of Sciences, Opatovický mlýn,
37901Třeboň, Czech republic
4 Institute of Physical Biology, University of South Bohemia, Zámek 136, 37333 Nové Hrady, Czech republic
Photosystem II (PSII) is a multisubunit
pigment-protein complex embedded in the thylakoid membranes of higher plants,
algae and cyanobacteria [1-3]. It performs series of photochemical reactions
resulting in the reduction of plastoquinone, the oxidation of water, and the
formation of a transmembrane pH gradient. The essential components of the PSII
complex are intrinsic membrane proteins that are almost identical between
cyanobacteria and higher plants: they include the D1 and D2 reaction center
proteins, chlorophyll a-binding proteins CP47 and CP43, α and β subunits of cytochrome b-559
(cyt b-559) and several low-molecular weight proteins with unknown
functions [3,4].
In
addition, there are extrinsic proteins associated with PSII, which play
important roles in maintaining the function and stability of the
oxygen-evolving complex [5]. As both cyanobacteria and higher plants
contain 33-kDa extrinsic subunit they differ in composition of the other
lumenal subunits. While higher plants and green algae contain the 23 and 16 kDa
extrinsic subunits, in cyanobacteria, these proteins are replaced by another
two proteins coded by psbU and psbV gene (cyt c550 and
12-kDa subunit) [6]. Red algal PSII complex has a 20 kDa protein in addition to
the three cyanobacterial proteins [7]. Recently, structure of the PSII complex isolated from two cyanobacterial
strains has been presented [8,9]. These models give the idea of the arrangement
of the intrinsic and extrinsic subunits. However, a location of the 20 kDa
extrinsic subunit within the red algal PSII is still unknown.
I this report we present preliminary results on
locating the the 20 kDa extrinsic protein using a transmission electron
microscopy and single particle image analysis of negatively-stained
preparations of Photosystem II isolated from a red alga Porhyridium
cruentum.
The PSII complex was isolated from thylakoid membranes from P. cruentum. Sucrose density gradient centrifugation of thylakoid membranes solubilized with β-dodecylmaltoside resulted in the separation of three green bands. On the basis of protein composition, absorption and 77K fluorescence emmision spectra, the lowest green band was used for further isolation of the PSII complex. This crude PSII extract were solubilized with β-dodecylmaltoside and loaded on DEAE-Sepharose CL-6B column according to [7]. The purified PSII complexes were eluted with 200 mM NaCl and analyzed.
Electron microscopy was performed with a Philips TEM 420 at magnification of 60,000 ´. The PSII complexes were applied on the glow discharged carbon-coated copper grids and negatively stained with 2% uranyl acetate. The image analysis was carried out with SPIDER software according to [10]. A total number of 253 single particle top-view projections were extracted from 14 negatively-stained electron microscopy images. The averaged top-view projection of PSII complex indicated a trapezoid particle with a dimension of 21 ´13 nm (Fig 1). Although no symmetry has been imposed during the image analysis clearly two-fold rotational symmetry around the center of the complex is visible indicating the dimeric nature of the PSII complex. This result is consistent with similar PSII preparations from both cyanobacteria and higher plants suggesting the PSII complex is structurally very similar in organism performing oxygenic photosynthesis [8,9, 11-13].
In order to investigate the organization of the extrinsic subunits (and mainly the location of 20 kDa protein) the PSII particles will be exposed to various salt treatments which remove the lumenal subunits. Thus, a difference map of averaged projections of PSII complexes with and without the extrinsic subunits would enable to determine the location of the 20 kDa subunit.
This work was
supported by grants LN00A141 and FRVS 1292/2002 of the Ministry of Education,
Youth and Sports of the Czech republic.
References:
1. Hansson O and Wydrzynski T (1990) Photosynth. Res. 23: 131-162
2. Ghanotakis DF. and Yocum CF (1985) Photosynth. Res. 7:
97-114
3. Barber J, Nield J, Morris EP, Zheleva D and Hankamer B (1997)
Physiol. Plant. 100: 817-827
4. Hankamer B, Morris EP, Nield J, Carne A and Barber J (2001) FEBS
Lett. 504: 142-51
5. Seidler A (1996) Biochim. Biophys. Acta 1277: 35-60
6. Shen JR, Ikeuchi M and Inoue Y (1992) FEBS Lett. 301:
145-149
7. Enami I, Murayama H, Ohta
H, Kamo M, Nakazato K and Shen JR (1995) Biochim. Biophys. Acta 1232:
208-216
8. Zouni A, Witt HT, Kern J,
Fromme P, Krauss N, Saenger W and Orth P (2001) Nature 409: 739-743
9. Kamiya N and Shen JR (2003) Proc. Natl. Acad. Sci. USA 100: 98-103
10. van Heel & Frank (1981) Ultramicroscopy 6:187-194
11. Boekema EJ, Hankamer B, Bald D, Kruip J, Nield J, Boonstra AF,
Barber J and Rogner M (1995) Proc. Natl. Acad. Sci. USA 92: 175-179
12. Nield J, Kruse O, Ruprecht J, da Fonseca P, Büchel C and Barber J
(2000) J. Biol. Chem. 275: 27940-27946
13. Hankamer B, Morris EP, Nield J, Gerle C and Barber J (2001b) J.
Struct. Biol. 135: 262-269
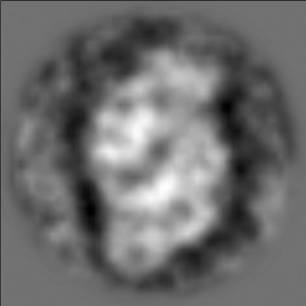
Figure 1. Averaged
projection of negatively-stained preparation of the dimeric PSII complex
isolated from Porhyridium cruentum in its top-view projection (i.e.
perpendicular view to the thylakoid membrane)