Crystallite size and microstrain in the structure of SrTiO3 formed by magnetron deposition with and without O2 flow through the deposition chambre
Zdeněk Jansa, Štěpánka Jansová, Lucie Nedvědová, Ján Minár
New Technologies Research Centre, University of West Bohemia in Pilsen, Pilsen
Perovskite compounds are ionic in nature. In the ABX3 formula above, A and B represent two cations, where A is a large cation and B is a medium cation. X is a small anion. The overall ionic structure must be neutral and therefore if we describe the charges on the individual ions qa, qb and qx, then the equation for the neutral configuration will be:
![]() [1]
[1]
One of the fields where the properties of Perovskites can and have already been applied is in the power industry. Experimental studies of the last few years have reliably demonstrated that, using simple modifications, perovskite oxides can be used in applications utilizing direct sunlight and photocatalytic applications. The original strontium titanate oxide SrTiO3 (abbreviated STO) is only able to use the UV component of incident radiation and is inactive in visible light, remaining transparent to visible light. The reason for this state is its wide band gap, which at room temperature has a value of 3.2 - 3.25 eV. Studies have shown that doping the structure of these oxides with transition metals (TM) can cause a shift in the valence and/or conduction band. This is because of the 3d dopant bands that create new energy levels in the mentioned band gap, effectively reducing it[2,3].
One of the elements of the TM group is nickel. According to some studies, it is likely that the Ni ion occurs in the cubic structure of STO in the form of Ni2+ and substitutes the Ti4+ sites. In the case of using this dopant, the shift of the absorption edge of STO:Nix relative to NiO was found to be 1.1 eV.
The present work investigates the differences in the structure of STO:Nix prepared by magnetron deposition method with different dopant amount settings. At the same time, the difference in structure was observed when prepared under vacuum with Ar working gas and when O2 was flowed through the deposition chamber. The premise of this experiment was to verify the formation of oxygen vacancies in the STO structure on which the photocatalytic phenomenon could occur[4,5].
All samples were prepared in a magnetron deposition chamber. The deposition chamber in the case of the first series was depleted to a base pressure of 2x10-4 Pa and filled with argon working gas. In the second case, the oxygen flow rate through the deposition chamber was set to 1.75 sccm. The samples are labeled A0 with O2 - sample without dopant used with O2 flow rate, A1 - sample with one Ni pellet, A1 with O2 - sample with one Ni pellet with O2 flow rate. Analogously, the designation is for samples with two and three Ni pellets.
The procedure of the experimental methods was identical for both series. After fabrication, all samples were first subjected to X-ray phase analysis. The samples were measured using both possible geometries available in the goniometer of the machine - the symmetric θ-θ geometry and the asymmetric ω-2θ geometry. In the initial stage, the samples were remeasured by GID in their original as-deposited state, then the samples were subjected to an in-situ experiment in a high-temperature chamber with a maximum temperature of 900°C.
The second experimental method used was scanning electron microscopy. The chemical composition of the samples and observation of the surface morphology of the samples and their fractures was performed on a JSM-7600F electron microscope from JEOL.
The third method was X-ray photoemission electron spectroscopy (XPS). The experiments on the selected samples were carried out in the working chamber. In this chamber, an ultra-high vacuum (base pressure ≤ 5×10-8 Pa) and a Phoibos 150 hemispherical analyzer were used throughout the experiments. The photon source was a Mg and Al X-ray tube.
Due to the demonstrated effect of O2 flow rate on the changes in diffraction line parameters, structural data were calculated for two samples of the second series (A2 and A2 with O2). The results for both samples, namely crystallite size and microdeformation, are graphically presented in Figures 1 a) and b). Here, a marked difference in the crystallite sizes can be seen.
a) 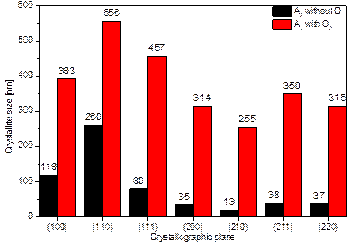 b)
b) 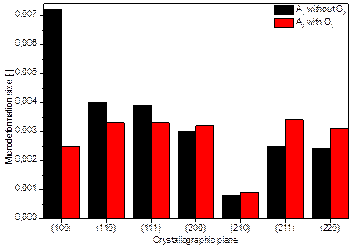
Figure 1. Graphical representation of the dependence of the amount of dopant and the presence of O2 flow of samples A2 and A2 with O2 on a) the size of the crystallites and b) the size of the microdeformations inside the lattice.
By evaluating the XPS spectra of samples A2, A2 with O2 and A3, it was found that the Ni dopant replaces titanium in the STO lattice, which is in the middle of the oxygen tetrahedron. In different ways, of course. As a result, ferroelectric phenomena can occur [1], which can be exploited for photocatalytic applications. In the case of comparison of A2 and A2 with O2, the difference in the measured data for the doping nickel is noticeable. Both samples have used the same amount of nickel pellets (2 pieces) during deposition, but in the case of sample A2 with O2, the air flow through the deposition chamber during deposition has been increased. Thus, the flowing oxygen obviously prevented the incorporation of nickel ions into the STO structure. From the measured data, it can be evaluated that the energies refer indeed to Ni-O bonding, which, however, occurs outside the doped STO:Ni structure. This conclusion is in agreement with the evaluated diffractogram of this sample, where the Ni(TiO3) phase was detected with the simultaneous formation of the Sr5Ni4O11 phase. And at the same time, this conclusion is supported by the findings from the SEM examination.
1. Richard J. D. Tilley, Perovskites: Structure-Property Relationships, First edition, 2016, ISBN: 978-1-118-93566-8.
2. A. Shah; Photovoltaic technology: The case for thin-film solar cells. Science, 285(5428): 692-698, jul 1999.
3. J. G. Bednorz and K. A. Mller, Perovskite-type oxides: The new approach to high-Tc superconductivity, Rev. Mod. Phys. 60, 585 (1988).
4. I. A. Sluchinskaya, A. I. Lebedev, and A. Erko, Structural position and charge state of nickel in SrTiO3, Phys. Solid State 56, 449 (2014).
5. S. K. Rout, S. Panigrahi, and J. Bera, Study on electrical properties of Ni-doped SrTiO3 ceramics using impedance spectroscopy, Bull. Mater. Sci. 28, 275 (2005).