Powder Diffraction and Solid Electrolytes, case of Metal Hydridoborates
R. Černý
DQMP, University of Geneva, Switzerland
Radovan.Cerny@unige.ch
Interest in metal hydrides was initially driven by the potential to develop efficient and safe on-board hydrogen stores working close to ambient pressure and temperature. In search for hydrides with higher gravimetric storage capacity, the researchers concentrated on hydrides based on light atoms, among others on Li and Na salts containing hydridoborate anions such as borohydride BH4− or closo-hydridoborate B12H122− [1]. The hydrogen absorption-desorption cycling in complex hydrides still needs more chemical ideas due to relatively strong covalent bonding. Unexpectedly, the high mobility of alkali metal cations in some complex hydrides has opened the door for their application as battery materials, mainly as solid-state electrolytes (SSE).
Replacing the liquid electrolyte by SSE offers several advantages: i) a solid material is more thermally stable, thus enhancing the overall safety of the battery; ii) being less prone to the dendrite penetration, it enables the use of alkali metals as negative electrodes and iii) acting as physical layer between the two electrodes, it has a beneficial effect on the cell performance [2].
Among the different classes of SSE, the metal hydridoborates have received particular interest, being soft, highly stable toward oxidation and exhibiting fast ion conductivity, enabled by an entropically-driven phase transition. Such transitions generally occur above room temperature (rt), and it is therefore necessary to frustrate the anionic lattice, for example by anion mixing or mechanical energy (Figure 1) to bring the superionic regime down to rt [3-8].
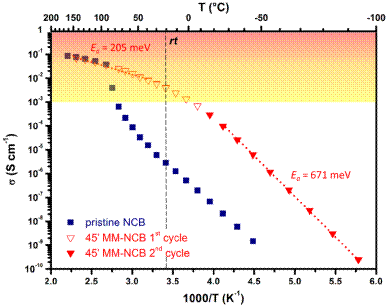
Figure 1. DC Conductivity evolution as function of the temperature for pristine (blue squares) and ball-milled-NaCB11H12 (red triangles). For the sake of clarity, the superionic regime (σ > 1 mS cm-1) has been highlighted in the graph.
The hydrogen storage and mobility of the cations in light complex hydrides depends on the structural features, pathways available in the anion packing and on the anion thermal motion. While the latter requires important experimental and theoretical effort, the first two parameters can be easily quantified from crystal structures obtained by X-ray powder diffraction (XPD). Main difficulties of crystal structure determination by XPD with hydridoborate samples are low crystallinity, overcome by global optimization (direct space) methods [9], and a multiphase character of the samples, solved by "decomposition aided indexing" [10] as was the case of CsCB11H12 [11] shown in Figure 2. The case of molecular compound C2B10H12 demonstrates the effect of inter-cluster interaction on stabilization of various crystal structure symmetries [12].
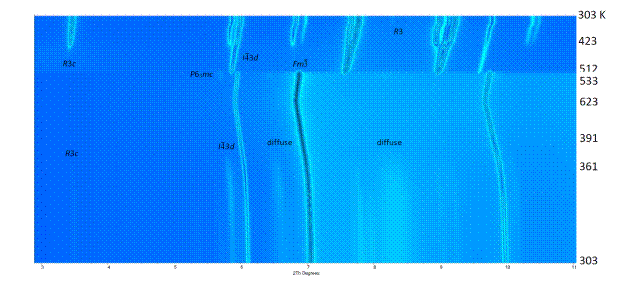
Figure 2. Temperature dependent synchrotron radiation XPD data measured on dry CsCB11H12 sample with fast heating/cooling rate of 10 K/min, wavelength l= 0.64113 Å. The peaks of different polymorphs are labelled with their space group symbols. Observed diffuse intensity is labelled too.
1. M. Paskevicius et al., Chem. Soc. Rev., 46, (2017), 1565.
2. W. Zeier, J. Janek, Nat. Energy, 1, (2016), 16141.
3. W.-S. Tang et al., ACS Energy Lett., 1, (2016), 659.
4. L. Duchêne et al., Energy Environ. Sci., 10, (2017), 2609.
5. F. Murgia et al., Electrochem. Comm., 106, (2019), 106534.
6. M. Brighi et al., Cell Reports Phys. Sci., 1, (2020), 100217.
7. M. Brighi et al., Adv. Materials Interfaces, 9, (2022), 202101254.
8. F. Murgia et al., Appl. Materials Interfaces, 13, (2021), 61346.
9. R. Černý, Crystals, 7, (2017), 142.
10. R. Černý, Y. Filinchuk, Z. Krist., 226, (2011), 882.
11. R. Černý et al., Molecules, 28, (2023), 2296.
12. M. Brighi et al., Inorg. Chem., 61, (2022), 5813.