Molecular simulation of interactions between palygorskite and TiO2.
Miroslav Pospíšil1, Aristos Mavrikos2, Eleni Gianni2, Christina-Vasiliki Lazaratou2, Milan Pšenička1 and Dimitrios Papoulis2
1Charles University, Faculty of Mathematics and Physics, Ke Karlovu 3, Prague 2, 12116, Czechia
2Department of Geology, University of Patras, Rio, 26504 Patras, Greece
miroslav.pospisil@mff.cuni.cz
TiO2 is very often used for photocatalytic applications because it is widely available, cheap and environmentally friendly photocatalytic material. Nevertheless, the anatase (preferred crystal structure) nanoparticles have tendency to agglomerate and this process causes a significant decreasing in catalysis efficiency. To avoid the agglomeration, we used the fibrous clay minerals such as palygorskite to anchor TiO2 particles among its fibres. This causes the photocatalytic properties of small nanoparticles to be preserved and no agglomeration due to the strong mutual interactions between TiO2 and clay fibres.
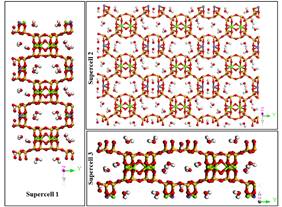
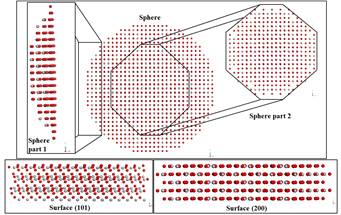
Palygorskite–TiO2 nanocomposites were prepared and characterized by various experimental techniques (X-ray diffraction, Fourier transform-infrared spectroscopy and transmission electron microscopy) in Greece, while their mutual interactions including probable spontaneous bonding between clay and TiO2 spheres were calculated by molecular simulation methods. Three different palygorskite supercells were built with different type of surface with the largest amount of oxygen atoms, see Figure 1, which were subsequently calculated in various positions with the TiO2 spheres with suitable adjacent surfaces, see figure 2.
|
|
|
Figure 1. The three supercells under different point of view. Supercell P1: A = 3, B = 1, C = 7 Supercell P2: A = 3, B = 3, C = 1 Supercell P3: A = 1, B = 2 and C = 7 [1]. |
Figure 2. The anatase (i) sphere part 1 and 101 and (ii) sphere part 2 and 2i the parts of anatase that were combined with palygorskite surface [1]. |
A monoclinic palygorskite structure was used for the calculations, the unit cell parameters of the crystal structure were as follows: a = 13.337 Å, b = 17.879 Å, c = 5.264 Å, α = γ = 90° and β = 105.270°, space group C2/m [2, 3]. An optimized crystal structure of TiO2, in the form of anatase, was imported from Materials Studio database with the following cell parameters: a = 3.776 Å, b = 3.776 Å, c = 9.486 Å, α = γ = β = 90°, and the space group number 141 and type I41/amd. A supercell of anatase was created with the following parameters: A = 20 a, B = 20 b, C = 10 c.
The simulations showed that optimal TiO2-palygorskite connection takes place among the Ti atoms of the curved TiO2 part of sphere with the O atoms of the P1 surface of palygorskite through strong electrostatic interactions and the possible formation of covalent bonds due to the short distance between the surfaces, see Table 1. Due to the simulation, we can see the detailed curved part of anatase with Ti atoms which closely interacting and nearly connected to the O atoms on various surfaces of palygorskite [1].
Table 1. Three shortest Ti-O distances of the selected optimized models [Å].
|
Name |
Distance 1 |
Distance 2 |
Distance 3 |
|
P11 |
2.748 |
2.909 |
3.143 |
|
P1101 |
2.123 |
2.185 |
2.198 |
|
P12 |
1.800 |
1.814 |
1.872 |
|
P12i |
1.790 |
2.050 |
2.627 |