Lipolytic system in the hard tick Ixodes ricinus
Tereza Kozelková1,2, Filip Dyčka2, Helena Frantová1, Veronika
Urbanová1,
and Petr Kopáček1*
1Institute of Parasitology, Biology Centre of the Czech Academy of Sciences, Branišovská 31, 370 05, České Budějovice, Czech Republic
2Faculty of Science,
University of South Bohemia, Branišovská 31, 370 05, České Budějovice,
Czech Republic
Ticks are obligatory blood-feeding ectoparasites capable of transmitting a wide variety of pathogens comprising bacteria, viruses, and protozoa to humans and animals. After mosquitoes, ticks are the second most dangerous vectors of arthropod-borne diseases. Hard tick Ixodes ricinus is a typical representative of the 3-host tick and its life cycle comprises three life stages. Each of the parasitic stages, except for adult males, feeds on a host. In contrast to mosquitoes, hard ticks I. ricinus feed much longer. Nymphal feeding takes typically from 3 to 4 days, adult females feed twice longer approximately 6 – 9 days. Adult ticks are capable to imbibe and digest huge amounts of host blood exceeding hundred times their unfed weight (Sonenshine, 1991).
Tick midgut lumen serves as the main organ for storage of the engorged blood. Most of the hematophagous ectoparasites (such as insect blood-feeders) digest host blood extracellularly in the midgut lumen. By contrast, digestion in ticks is a slow process occurring intracellularly in the midgut epithelium cells. Host blood is the sole source of energy and nutrients for overall tick development and reproduction. During feeding of each developmental stage, dynamic changes of tick midgut epithelium reflect the changes in the physiological processes occurring in this tissue (Sonenshine, 1991). Furthermore, the midgut serves as the primary interface between the tick and tick-borne pathogens that determines tick vector competence (O'Neal et al., 2020).
Limited information and functional studies about vector insect lipid metabolism are available. Lipids, in the form of sterols or free fatty acids, are the main and essential components of the insect dietary requirements (Canavoso et al., 2001; Toprak et al., 2020). However, insects are not able to synthesize sterols by themselves (Clark and Block, 1959; Jing and Behmer, 2020). During oxidation of the fatty acids (FAs), twice more energy (approximately 9kcal/g) is released than during the complete oxidation of carbohydrates (approximately 4kcal/g) (Toprak et al., 2020). The main source of lipids for these invertebrates is host blood. Lipid digestion occurs mainly in the midgut lumen, where lipases (EC 3.1.1.3; the major lipid digestive enzymes) catalyze the hydrolysis of the ester bond in triacylglycerols (TAGs) as well as in di- and mono- acylglycerols (DAGs, MAGs) to the final products - glycerol and free fatty acids (FAs) (Derewenda, 1994; Toprak et al., 2020).
In I. ricinus, poor understanding of lipid metabolism exists. As mentioned above, digestion of proteins in tick midgut occurs intracellularly in the midgut epithelium cells. Nothing is known about digestion of lipids in ticks and no digestive lipases have been yet functionally characterized in any tick species. Some esterases and lipases involved in the incorporation of the nutrients to the oocytes were identified during embryogenesis in the eggs of the camel tick Hyalomma dromedarii (Fahmy et al., 2004). Earlier, Koh et al. (1991) described the utilization of the nutritional reserves stored in the form of lipid droplets in the midgut epithelium cells. These lipids droplets are used for the growth of tissues during feeding in the nymphal stage of hard tick Haemaphysalis longicornis (Koh et al., 1991). More recently, it was demonstrated in another tick species Dermacentor variabilis, that lipids are also exploited in the course of the long off-host starvation period, during which the large lipid droplets are released and used as the endogenous energy recourses (Rosendale et al., 2018). In the midgut transcriptome of I. ricinus, lipases from several classes have been identified (Perner et al., 2016). Phospholipase A2, acid sphingomyelinase, and lipase (pancreatic-like type) were identified as the most up-regulated lipases in the midgut on the 3rd day of adult female feeding (Perner et al., 2016). Detailed analysis of these midgut-specific lipases using quantitative real time PCR (qRT-PCR) across tick development revealed that these lipases are mainly expressed during the early stage of adult female feeding (Fig. 1A-C). The RNAi mediated-knockdown (RNAi KD) of individual lipases in adult females did not show any phenotype differences between RNAi KD and control groups (data not shown). Furthermore, in the midgut proteome analysis, either form unfed females or from females fed for five days, only rare lipases were identified (Fig. 1D).
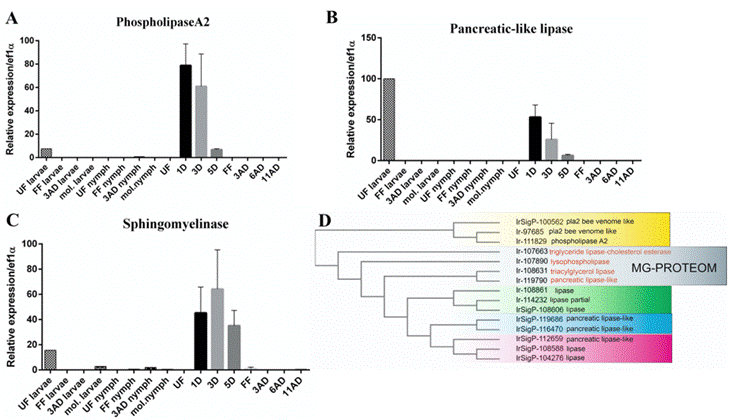
Figure 1: (A-C) - mRNA expression of individual lipases in the midgut during developmental and feeding stages. Quantitative real-time PCR (qRT-PCR) profiling of the phospholipase A2 (PlA2), pancreatic-like lipase, and sphingomyelinase. Bars indicate the standard deviation. UF: unfed; 1D,3D, 5D: one, three and five days of feeding; FF: fully fed females; 3AD, 6AD, 11AD: three, six and eleven days after detachment; mol.: molting. (D) – Phylogenetic three of lipases identified in the midgut transcriptome (Perner et al., 2016a) and in the midgut proteome. Lipases identified in the midgut proteome are highlighted grey. Phylogenetic tree was performed using ClustalOmega server (https://www.ebi.ac.uk/Tools/msa/clustalo/).
Acknowledgement: Supported by GACR 21-08826S and ERDFunds (No.CZ.02.1.01/0.0/0.0/16_019/0000759)
Canavoso, L.E., Jouni, Z.E., Karnas, K.J., Pennington, J.E., Wells, M.A. (2001). Fat metabolism in insects. Annu Rev Nutr 21, 23-46.
Clark, A.J., Block, K. (1959). The absence of sterol synthesis in insects. J Biol Chem 234, 2578-2582.
Derewenda, Z.S. (1994). Structure and function of lipases. Adv Protein Chem 45:1-52.
Fahmy, A.S., Abdel-Gany, S.S., Mohamed, T.M., Mohamed, S.A. (2004). Esterase and lipase in camel tick Hyalomma dromedarii (Acari: Ixodidae) during embryogenesis. Comp Biochem Physiol B Biochem Mol Biol 137, 159-168
Jing, X., Behmer, S.T. (2020). Insect Sterol Nutrition: Physiological Mechanisms, Ecology, and Applications. Annu Rev Entomol 65, 251-271.
Koh, K., Mori, T., Shiraishi, S., Uchida, T.A. (1991). Ultrastructural changes of the midgut epithelial cells in feeding and moulting nymphs of the tick Haemaphysalis longicornis. Int J Parasitol 21(1):23-36.
O'Neal, A.J., Butler, L.R., Rolandelli, A., Gilk, S.D., Pedra, J.H. (2020). Lipid hijacking: a unifying theme in vector-borne diseases. Elife 9.
Perner, J., Provazník, J., Schrenková, J., Urbanová, V., Ribeiro, J.M., Kopáček, P. (2016). RNA-seq analyses of the midgut from blood- and serum-fed Ixodes ricinus ticks. Sci Rep 6:36695.
Rosendale, A.J., Dunlevy, M.E., McCue, M.D., Benoit, J.B. (2018). Progressive behavioural, physiological and transcriptomic shifts over the course of prolonged starvation in ticks. Mol Ecol 28(1):49-65.
Sonenshine, D.E. (1991). Biology of ticks. Oxford University Press, New York. First ed., Vol. 1.
Toprak, U., Hegedus, D., Dogan, C., Guney, G. (2020). A journey into the world of insect lipid metabolism. Arch Insect Biochem Physiol 104, e21682.