Determination of structure of small particles
P. Roupcová1,2, O. Schneeweiss1, T. Sojková1, N. Pizurová1
1Institute of Physics of Material ASCR, Zizkova 22, Brno 61662, Czech Republic
2CEITEC Brno University of Technology, Purkyňova 123, Brno 612 00, Czech Republic
roupcova@ipm.cz
We are producing and studying Magnetic Nanoparticles (MNPs) due its applications in biomedicine. The suitable size have to be comparable to biological entities (cells, proteins, and genes), controllable transport of MNPs in human body (drug delivery). The purpose of our study is produce particles for their ability to generate heat when an AC magnetic field is applied (magnetic hyperthermia). In particular, magnetic hyperthermia therapy is based on the fact that some types of cancer cells are more sensitive at temperature 41-45 °C than the healthy cells and that the required heat can be produced by MNPs. Nowadays majority in-field investigations are based on in vitro or in vivo animal model, but also, in the case of iron oxide based MNPs, this approach is used at the clinical level [1]. The heating ability of MNPs is dependent on morphology, microstructural and magnetic properties of MNPs, but also related to the amplitude and frequency of an applied magnetic field. In that sense, during the last years the synthesis methods have been intensively developed in order to control particle size distribution, surface effects and the degree of interparticle interactions, so that magnetic properties favourable for particular application could be successfully tailored. Although the tons of studies was published, there is huge confusion and misunderstanding in terms such as particle, crystalline and grain size, which influenced the main characteristic – the magnetic properties. The confusion is originated by very wrong understanding of analytical method which are applied. For our purpose of magnetic hyperthermia, we are looking for magnetite Fe3O4 and maghemite γ-Fe2O3.
The standard method of determination of phase composition by X-ray powder diffraction (XRD) is not very helpful (see Fig. 1) in our case it produced results on the range in between nano/amorphous which could be interpreted in any way or it could not be interpreted at all.
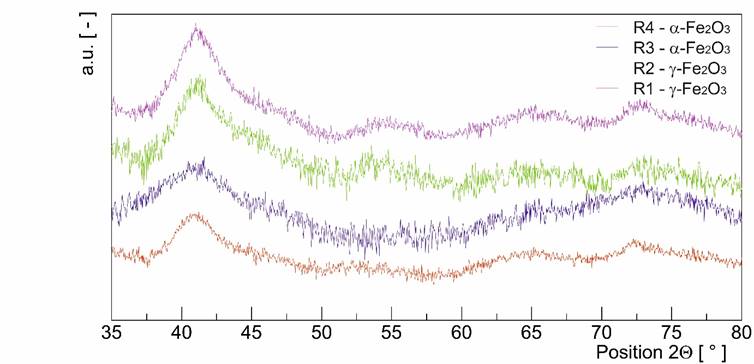
Figure 1. X-ray pattern of tiny particles.
As well as the chemical composition obtain by EDS do not lead us to the correct results because the presence of S was overlooked in the samples R1 a R2. Eventually, the real structure and the chemical bound was determined by X-ray photoemission and Mössbauer spectroscopy which show as the presence of S and omit the existence Fe2+ type of bound for all samples. The measurement of magnetic properties by PPMS was much more helpful. It distinguish Neél temperatures and Morine transition of hematite (R3 and R4) at 940K and at 250K and the same temperatures when maghemite transform to hematite (R1 and R2) [2,3].
This more advanced technique helps us to determine the phase composition as maghemite and hematite instead of magnetite and maghemite, we are expecting.
1. M.R. Horsman, J. Overgaard, Clinical Oncology, 19, (2007) 418.
2. Cornell, R. M., Schwertmann, The Iron Oxides, Weinheim, Germany, WileyVCH, 2003, 664.
3. MORIN, F., Physical Review, 78 , (1950) 819.
We acknowledge CzechNanoLab Research Infrastructure supported by MEYS CR (LM2018110).