Hydrothermal conversion of Cerium Oxalate into CeO2.nH2O oxide
N.Assi, V.Tyrpekl
Department of Inorganic Chemistry, Faculty of Science, Charles University, Hlavova, 2030 Prague,
Czech Republic
1. Introduction
In the last few decades, various methods were applied for the synthesis of micro and nano structures of metal oxide which consist of precipitation, combustion, ionic liquid route, and sol-gel. The inconveniences presented in these methods such as pH control, the high temperature used, long reaction time and low purity, encouraged the researcher to find the suitable substitution methods. Thermal decomposition methods could be employed as an alternative approach. The utility of thermal decomposition for inorganic salts such as hydroxide, carboxylate, acetate, and oxalate has been extensively used to obtain metal oxides [1].
Oxalate hydrate could be a good precursor for the preparation of metal oxide due to its large quantity mass loss in the hydrothermal process (more than 50 wt %), low cost, easy synthesis, and low decomposition temperature in the air. Indeed, the hydrothermal decomposition of metal oxalates is a clean, flexible and powerful approach toward metal oxide with possible scale-up potential [2].
Metal oxalate hydrates can be dehydrated and decomposed by pressure and heating and finally converted to metal oxide. Ergo, metal oxalate hydrates have been exploited as a precursor to convert metal oxides with solid-state reaction methods [3].
In this study, we prepared a ceria solid solution with simple oxalate precipitation [4]. The obtain powder which consist Ce2(C2O4)3.10H2O was used for conversion to CeO2 with hydrothermal treatment. The impact parameters such as pH, and hydrothermal temperature were evaluated on the physicochemical characterization of the final powder.
2. Experimental and Instrumental section
All the reagents used in this work were analytical grade and all solutions were prepared with Millipore water. Briefly, appropriate amount of oxalic acid (0.1 M) add to the cerium(III) nitrate hexahydrate (0.1 M) mixed together. The white precipitation was washed 3 times with Millipore water, centrifuged and dried at 50 °C in an oven. 0.25 g of the obtained powder with 21 mL of diluted nitric acid was transferred to the Teflon-lined autoclave for hydrothermally treatment. The final product washed 3 times with Millipore water, centrifuged and dried at 50 °C in an oven. The final powder was characterized with XRD and SEM.
3. Result and Discussion
Several parameters are affected to synthesis metal oxide from metal oxalate as precursor. The pH value is the significant key factor for the nature and crystallinity [5]. Therefore, the influence of the initial pH during the Ce2(C2O4)3.10H2O hydrothermal treatment was investigated in the 1, 2 and 3. The target pH was reached by adding different concentration of nitric acid. The XRD patterns of the final powder prepared in 220 °C at different adjusted pH for 24 h are shown in the Fig. 1
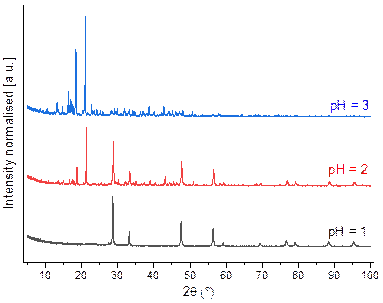
Figure 1. Powder X-ray diffraction diagrams obtained after hydrothermal treatment of Ce2(C2O4)3.10H2O in variation initial pH in 220 °C for 24 h.
Other main effective parameter on the materials physicochemical properties is temperature [8]. Therefore, the effect of hydrothermal treatment for converting Ce2(C2O4)3.10H2O to CeO2 was evaluated at 180, 200 and 220 °C with adjusted pH 1 for 24 h. As the XRDs are illustrated in the Fig. 2 with increasing hydrothermal temperature the initially precipitated of Ce2(C2O4)3.10H2O converted to CeO2.The optimum temperature for the conversion is 200 °C and in higher temperature it just lead to sharp peaks and consequently growing crystallise structure.
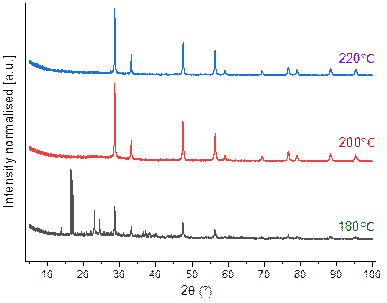
Figure 2. Powder X-ray diffraction diagrams obtained after hydrothermal treatment of Ce2(C2O4)3.10H2O with various temperatures in pH 1 during 24 h.
Final structure such as size, crystal thickness, aggregation and purity are related to the synthesis condition, as confirmed by our achievement. However, Fig. 3 shows the size and morphology of the achieved powder from hydrothermal treatment of Ce2(C2O4)3.10H2O to the CeO2. Such modification of this conversion was correlated to increase crystallite phase with temperature and pH as already evidenced by XRD measurements. As it illustrated in Fig. 3 (a-c) with increasing the temperature from 180 to 220 °C aggregation of the nano powder sticking together to formation bigger structure was occurred. Also with increasing pH from 1 to 3, gradually conversion of Ce2(C2O4)3.10H2O to the CeO2 was decreased and porous powders in the low pH change to the rods and it shown in Fig. 3 (c-e).
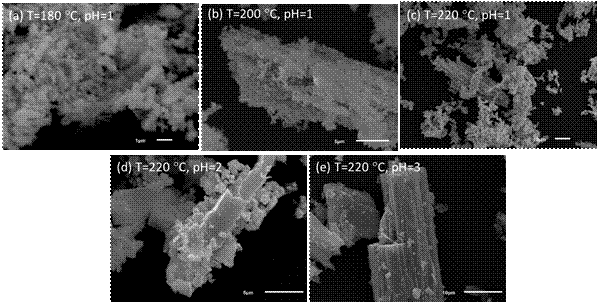
Figure 3. SEM images of Ce2(C2O4)3.10H2O conversion to CeO2 in different hydrothermal treatment (a-c) and pH (c-e) during 24 h.
4. Conclusion
Cerium (III) precipitated with oxalic acid in the aqueous solution and the white powder of Ce2(C2O4)3.10H2O was achieved. Entirely conversion of Ce2(C2O4)3.10H2O to the CeO2 was done with hydrothermal treatment. This conversion was profoundly related to several parameters such as pH solution and temperature in the process of hydrothermal treatment which optimized in this procedure.
References
1. L. Guo, H. Arafune, N. Teramae, Langmuir., 29, (2013), 4404.
2. O. Walter, K. Popa, O.D. Blanco, Open Chemistry, 14, (2016), 170.
3. C. Hu, J. Mi, S. Shang, J. Shangguan, J. Therm. Anal. Calorim., 115 (2014), 1119.
4. V. Tyrpekl, P. Markova, M. Dopita, P. Brázda, M. A. Vacca, Inorg. Chem., 58, (2019),10111.
5. A. I. Y. TokF, Y. C. Boey, Z. Dong, X. L. Sun, J. MATER. PROCESS. TECH.,190, (2007), 217.
8. J. Manaud, J. Maynadié, A. Mesbah, M. O. J. Y. Hunault, P. M. Martin, M. Zunino, N. Dacheux, N. Clavie, Inorg. Chem., 59, (2020), 14954.