Temporal evolution of optical absorption and emission spectra of thiol capped CdTe quantum dots
S. Gupta1, S. Tomar2, 3, A. Priyam4, B. Bhushan5, A. Singh6, U. K. Dwivedi7, R. K. Choubey2
1Department of Condensed Matter Physics, Faculty of Mathematics and Physics, Charles University, Ke Karlovu 5, Prague- 12116, Czech Republic
2Department of Physics, Amity Institute of Applied Sciences, Amity University, Noida Sector 125, Uttar Pradesh- 201313, India
3Applied Science and Humanities Department, ABES Engineering College, Campus- 1, 19thKM stone NH- 24, Ghaziabad, Uttar Pradesh- 201009, India
4Department of Chemistry, School of Physical and Chemical Sciences, Central University of South Bihar, SH- 7, Gaya-Panchapur Road, Gaya, Bihar- 824236, India
5Department of Physics, School of Applied Sciences, Kalinga Institute of Industrial Technology, Bhubaneshwar, Odisha- 751024, India
6Department of Physics, Faculty of Natural Sciences, Jamia Millia Islamia, Central University, New Delhi- 110025, India
7Department of Applied Physics, Amity School of Applied Sciences, Amity University Jaipur, Rajasthan- 303002, India
e-mail: suhaas96@gmail.com
Quantum dots (QDs) are strongly confined semiconductor nanoparticles that exhibit novel and size-tunable properties as a result of quantum confinement effects in the particle size regime comparable to their Bohr excitonic radius. QDs have been rigorously studied to employ their desirable properties in optoelectronic devices; these very same size-tunable optoelectronic properties have also been investigated for potentially widespread use in sensing and imaging applications in biological systems [1-3]. CdTe is such a II-VI semiconductor compound, whose particle size can be tailored to exhibit luminescence across the visible spectrum [4]. However, to make CdTe a suitable material to be used in biological systems, it must be made water-soluble with the use of a biocompatible ‘capping’ material; capping the CdTe QD also affords it increased mechanical, chemical and luminescent stability [5]. The choice of functional group of the material used to cap the CdTe QD also lends to it easily tuneable surface properties that can be used in biomedical tracking and drug delivery applications [6].
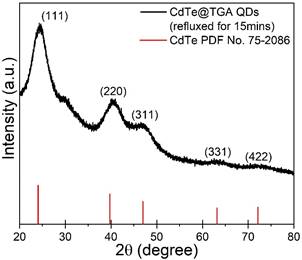
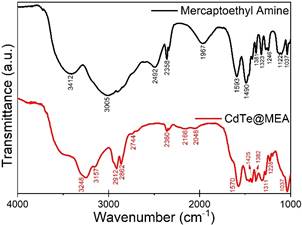
In the present work, we have synthesised and studied a series of CdTe QDs capped with four different thiols: (i) Thioglycolic acid (TGA), (ii) Mercaptoethyl amine (MEA), (iii) L-Cysteine (L-Cys), and (iv) Glutathione (GSH). An aqueous route was employed to synthesise all the samples, with varying reflux times for the different thiols used for capping. Fig.1 illustrates the confirmation of the cubic CdTe phase of the seed material from the XRD analysis of the synthesised CdTe@TGA QDs refluxed for 15mins; highest intensity characteristic (111) peak was further analysed to calculate the lower limit of crystallite size (2.4nm), interplanar spacing (3.65Å), lattice constant (6.32Å), microstrain and dislocation density. HRTEM and SAED studies further supported the results obtained from the XRD analysis. Fig.2 shows the comparison of the FTIR spectra of the CdTe@MEA QDs with the FTIR spectra of MEA; evidence of cleavage of the H-SR bond in thiols and simultaneous formation of the Cd-SR bond was used to confirm the successful capping of all the thiols to the surface of the CdTe QDs.
|
|
|
|
Figure 1. XRD analysis of the synthesised CdTe@TGA QDs refluxed for 15mins |
Figure 2. Comparison of the FTIR spectra of the synthesised CdTe@MEA QDs with the FTIR spectra of MEA |
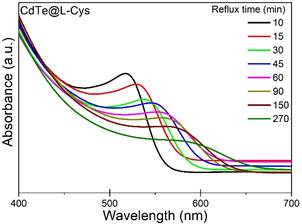
Fig.3 shows the UV-visible absorption spectra of the synthesised CdTe@L-Cys QDs refluxed for different times. All synthesised samples exhibited blue-shift in absorption edge as compared to the bulk CdTe, confirming the strong size confinement of the nanoparticles; with increasing reflux time, the absorption edge shifted gradually to longer wavelengths for all samples. Band-gap energy was obtained from the Gaussian-fitted peak position of the 1st derivative of absorption v/s energy plot; tight binding approximation model was used to calculate the size of the QD from the band gap energy. Particle size growth with increasing reflux time was observed for all samples: CdTe@GSH reached a predetermined particle size (4nm) the fastest (90mins) when compared to the other thiol capped CdTe QDs, while the CdTe@MEA QDs were unable to attain the same particle size even after 390mins of reflux time. To understand the growth mechanics, variations of concentration and particle size distribution of the synthesised thiol-capped CdTe QDs with respect to increasing reflux time and particle size was studied. Evidence of separation between the nucleation and growth processes, and identification of three distinct growth regimes (focusing, defocusing and equilibrium) allowed us to confirm the dominance of Ostwald ripening processes during the particle growth.
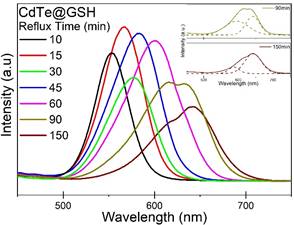
Fig.4 shows the photoluminescent emission spectra of the synthesised CdTe@GSH QDs refluxed for different times. At shorter reflux times, all samples exhibit narrow-width single peak emission, which gradually shifts to longer wavelengths, from green to yellow to orange to red, with increasing reflux time and particle size. At longer reflux times, deconvolution of the emission peak into Gaussian components was done to qualitatively investigate the contribution of emission from different sized particles, as can be seen in the inset of Fig.4; comparison of the component peaks at different times suggests that as reflux time increases, emission intensity contribution from larger particle sizes increases. Zetapotential analysis was conducted to obtain the surface charge of the synthesised thiol-capped CdTe QDs to study the effect the different functional groups on the surface of the QD; DLS analysis was conducted to obtain the hydrodynamic diameter of the synthesised thiol-capped CdTe QDs.
|
|
|
|
Figure 3. UV-visible absorption spectra of the synthesised CdTe@L-Cys QDs refluxed for different times |
Figure 4. Photoluminescent emission spectra of the synthesised CdTe@GSH QDs refluxed for different times. Inset shows the deconvolution of the emission peaks at longer reflux times. |
1. I. L. Medintz, H. T. Uyeda, E. R. Goldman, H. Mattoussi, Nature Materials, 4(6), (2005), 435.
2. X. Gao, Y. Cui, R. M. Levenson, L. W. Chung, S. Nie, Nature Biotechnology, 22(8), (2004), 969.
3. C. Y. Zhang, H. C. Yeh, M. T. Kuroki, T. H. Wang, Nature Materials, 4(11), (2005), 826.
4. D. V. Talapin, S. Haubold, A. L. Rogach, A. Kornowski, M. Haase, H. Weller, The Journal of Physical Chemistry B, 105(12), (2001), 2260.
5. N. Gaponik, A. L. Rogach, Physical Chemistry Chemical Physics, 12(31), (2010), 8685.
6. L. Qi, X. Gao, Expert Opinion on Drug Delivery, 5(3), (2008), 263.