TEMPERATURE INDUCED
STRUCTURAL EVOLUTION
OF POROUS IMIDAZOLATE COMPOUNDS VIA SYNCHROTRON XRPD
Sanja Burazer1,
Milan Dopita1, Yaroslav Filinchuk2,
Radovan Černy3, Jasminka Popović4
1 Department of Condensed Matter Physics, Faculty of Mathematics and
Physics, Charles University, Prague, Czech Republic
2 Institute of Condensed Matter and Nanosciences,
Université catholique de
Louvain, Louvain-la-Neuve, Belgium
3 Laboratory of Crystallography, DQMP, University of Geneva,
Geneva, Switzerland
4 Laboratory for Synthesis and Crystallography of Functional
Materials, Division for Materials Physics, Ruđer Bošković Institute, Zagreb, Croatia
Zeolitic imidazolate frameworks (ZIFs) are
an interesting class of metal-organic frameworks, structured by tetrahedrally
configured transition metal cations bridged by imidazolate (Im).
ZIFs are able to reproduce the zeolitic topology but
also incorporate the electronic properties of the transition metal ions.[1]
In this research, novel high-temperature
polymorph of sodium imidazolate, HT-NaIm was
discovered. Solid-state NMR was used for initial elucidation of structural
features, the crystal structure was
determined by single-crystal X-ray diffraction, while the in-situ HT-XRPD experiments utilizing synchrotron radiation have
been performed in order to gain the insight into the structural evolution and
thermal stability which was additionally analized by
differential thermal analysis and hot stage microscopy measurements. HT-NaIm exhibits pores of 50 Å3 that suggest
possible application for gas sorption/separation. Once formed, high-temperature polymorph of NaIm retains its structure and remains stable at room
temperature, what is important application-wise.
Additionally, new family of mixed
bimetallic imidazolates AMIm3 (A=Na, K; M=Mg, Mn) has been
synthesized and crystal structures were determined from powder X-ray
diffraction data. Temperature aided decomposition during in-situ SR HT-XRPD experiments gave the information about
structural changes ad thermal stability of the prepared samples. All compounds
have the imidazolate ligand connected to four metal cations forming a complex
3D network with channels running along the c-direction,
thus showing the similar sorption potential because of the empty volume of
around 30 Å3 incorporated inside the channels (Figure 1).[2]
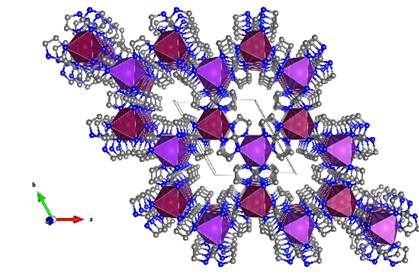
Figure 1. Extended crystal packing of KMgIm3 showing channels along
the c-direction.
1. O. M. Yaghi, M. J. Kalmutzki, and C. S. Diercks. in Introduction to Reticular Chemistry: Metal-Organic
Frameworks and Covalent Organic Frameworks (Wiley-VCH Verlag GmbH & Co.
KGaA), 2019,
463-479.
2. S. Burazer, F. Morelle, Y. Filinchuk, R. Černý, J. Popović, Inorganic Chemistry, 58, (2019)
6927-6933.
The
authors acknowledge the Swiss-Norwegian Beamlines of ESRF for the allocation of
beamtime and excellent support with the data collection.