Study of arrangement of intercalated organic species within layered materials by molecular simulation methods
J. Škoda, M. Pospíšil, P. Kovář
Charles University, Faculty of Mathematics and Physics, Ke Karlovu 3, 121 16 Prague 2, Czech Republic
kuba.skoda@gmail.com
We used classical molecular simulations to study the arrangements of various intercalated organic species (i) optically active 4-4’-dipyridylamine derivatives [1] within the layers of zirconium 4-sulfophenylphosphonates (ZrSPhP) and (ii) molecules of anti-inflammatory drugs within the layers of Mg2Al layered double hydroxides (LDH). We present the arrangement of the intercalated molecules of 3‑methoxo-N-(pyridin-4-yl)pyridin-4-amine (moAPY2, see Fig. 1) between the layers of ZrSPhP and mefenamic acid (see Fig. 2) intercalated within the layers of LDH. Their mutual positions and various orientations were calculated by the molecular simulation methods with the respect to the lowest value of the total potential energy.
|
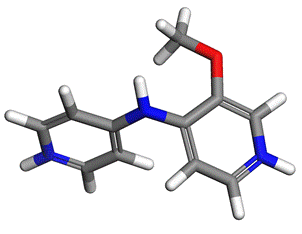
Figure 1. Molecule of 3 methoxo-N-(pyridin-4-yl)pyridin-4-amine (moAPY2) |
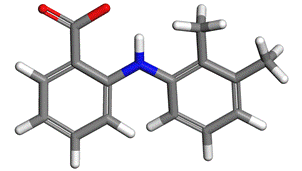
Figure 2. Molecule of mefenamic acid |
For the case of ZrSPhP, the simulations revealed that the alternating orientation of the intercalated moAPY2 molecules is preferred. The best calculated model is shown in the Fig. 3. The intercalate is characterized by disordered arrangement where at several places the intercalated molecules even trench on the sulfo groups’ region. In all initial models, the intercalated molecules were arranged in rows in each interlayer, variously perpendicularly or parallel to the cell axis a. The calculation showed that one orientation of the rows is not favoured as the best model includes both types of those arrangements in two interlayers. The intercalated molecules are stacked together in the optimized arrangement by the interactions between the pyridine rings.
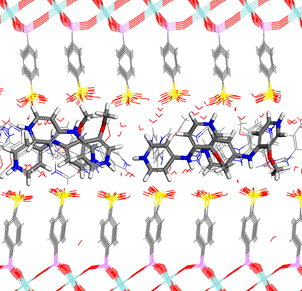
Figure 3. Optimized arrangement of the intercalated ZrSPhP with moAPY2 molecules, side view
Regarding the intercalation of LDH, molecules of mefenamic acid as drug with anti-inflammatory effect were inserted between the layers of Mg2Al LDH together with chlorine anions, in accordance to the chemical analysis [2]. From different types of arrangement of the intercalated molecules, the lowest value of potential energy and the best agreement with experimental XRD pattern [2] was reached for the bilayer arrangement, shown in Fig. 4. Due to the electrostatic interactions, the chlorine anions are closely attached to the layers of positively charged hydroxides and the COO- functional groups of mefenamic acid molecules are turned to the LDH layers.
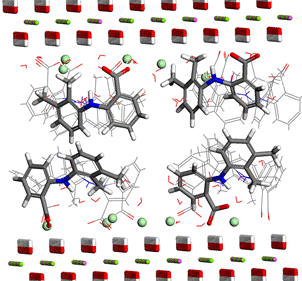
Figure 4. Optimized arrangement of the intercalated layered double hydroxides with molecules of mefenamic acid
1. F. Bureš, D. Cvejn, K. Melánová, L. Beneš, J. Svoboda, V. Zima, O. Pytela, T. Mikysek, Z. Růžičková, I. V. Kityk, A. Wojciechowski, N. AlZayed, J. Mater. Chem. C, 4 (2016), 468.
2. V. R. R. Cunha, V. A. Guilherme, E. de Paula, D. R. de Araujo, R. O. Silva, J. V. R. Medeiros, J. R. S. A. Leite, P. A. D. Petersen, M. Foldvari, H. M. Petrilli, V. R. L. Constantino, Mat. Sci. Eng. C 58, (2016) 629.
The study about LDH intercalation with drugs was supported by the Charles University, project GΑ UK No. 194217.