Solvatomorphism of nickel(II) complex with salen-type ligand
A. Vráblová1,3, L. R. Falvello2, M. Tomás3, J. Černák1
1Department of Inorganic Chemistry, Institute of Chemistry, P. J. Šafárik University in
Košice, Moyzesova 11, Košice, SK-04154, Slovakia
2Department of Inorganic Chemistry and ICMA, University of Zaragoza - CSIC, Pedro
Cerbuna 12, Zaragoza, E-50009, Spain
3Institute of Chemical Synthesis and Homogeneous Catalysis (ISQCH), University of
Zaragoza
Solvatomorphism is an important phenomenon with respect to many areas of chemistry and pharmacy [1,2]. This phenomenon was also observed for various coordination compounds [3], among others also in the case of Schiff base type complexes [4]. Schiff base H2(o-van-en) see scheme below) was formed by the condensation reaction of o-vanillin and ethylenediamine.
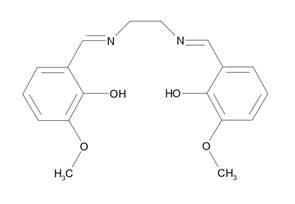
After deprotonation it can act as a ditopic ligand with one smaller coordination site formed by two nitrogen and two hydroxy oxygen atoms (donor set O2N2) preferably occupied by a 3d metal, while the larger coordination site formed by four oxygen atoms may accommodate, among others, a larger 4f atom [5] or, if it remains unoccupied by a metal atom, it is open for intermolecular interactions. Reaction of H2(o-van-en) with nickel carbonate yielded the Ni(II) complex [Ni(o-van-en)]·H2O (1) in microcrystalline form. Recrystallization of 1 from various solvents, with the aim of preparing single crystals, yielded single crystals of solvatomorphs [Ni(o-van-en)] ·H2O (1, recrystallization from acetone), [Ni(o-van-en)] ·H2O ·EtH (2, recrystallization from ethanol) and [Ni(o-van-en)] ·H2O ·iPrOH (3, recrystallization from isopropanol). While the hydrate 1 was already structurally characterized [6], the other two solvatomorphs 2 and 3 are novel. Incorporation of different solvate molecules in the respective crystal structures causes not only significant modification of some geometric parameters of the complex molecules but also evokes marked differences in the resulting supramolecular structures of the respective solvatomorphs.
We thank APVV project (APVV-0078-14) and the grant MAT2011-27233-C02-01 (Spain) for financial support.
1. H.G. Brittain, J. Pharm. Sci., 101 (2012) 464-484.
2. S. Boothroyd, A. Kerridge, A. Broo, D. Buttar, J. Anwar, Cryst. Growth Des., 18 (2018) 1903-1908.
3. O. Y. Vassilyeva, K. V. Kasyanova, V. N. Kokozay, B. W. Skelton, Acta Cryst. (2018). E74, 1532–1535.
4. J. Sirirak, D.J. Harding, P. Harding, L.J. Liu, S.G. Telfer, Austr. J. Chem., 68 (2015) 766-773.
5. C. Takehara, P. L. Then, Y. Kataoka, M. Nakano, T. Yamamura, T. Kajiwara, Dalton Trans. 44 (2015) 18276-18283.
6. D. Cunningham, J. F. Gallagher, T. Higgins, P. McArdle, J. McGinley, M. O'Gara, J. Chem. Soc., Dalton Trans., (1993) 2183-2190.