Crystal structure of edaravone cocrystals
S. Matejová1, V. Jorík1, M. Veverka2, M. Koman1
1Department of Inorganic Chemistry, Faculty of Chemical and Food Technology, Slovak University of Technology, Radinského 9, 812 37 Bratislava, Slovakia
2Research and Development Department, Eurofins Bel/Novamann Ltd., Kollárovo nám. 9, 811 07 Bratislava, Slovakia
simona.matejova@stuba.sk
We have focused on characterization of cocrystallization products of edaravone, a neuroprotective agent, and phenolic acids in order to prove the formation of cocrystals. New derivates of edaravone were prepared because it could improve physicochemical properties of this drug.
The goal of qualitative phase analysis is to determine what phases are present in sample, such as reactants, cocrystals with different stoichiometric ratios of starting materials and their polymorphs and pseudopolymorphs.
The preparation of cocrystals was performed, with tree experimental procedures, namely solvent drop grinding, solvent cocrystallization and slurring method at 1:1, 2:1, and 1:2 molar ratios with different solvents. Mainly, powder samples were prepared.
As cocrystals differ from salts or continuum salt-cocrystal only by the position of proton between the acidic and the basic functionality of the co-crystallization components, we can decide about the form of the products only after determination of their crystal structure. The position of the proton was determined indirectly by deducting it from the bond lengths C-O and C=O groups of carboxyl groups.
More then forty powder samples
have been characterized, out of which ten pure crystalline products have been
confirmed. Preliminary crystal structures of three products have been solved (Table).
Crystal structure of cocrystal Edaravon: Camphanic acid 2:1 (Fig. 1)
and cocrystal Edaravon:
4-Sulfobenzoic acid 1:2 (Fig. 2) have been solved by powder
diffraction analysis. Continuum salt - cocrystal Edaravon: Trimesic acid 1:2 (Fig. 3)
has been solved by a single crystal diffraction analysis.
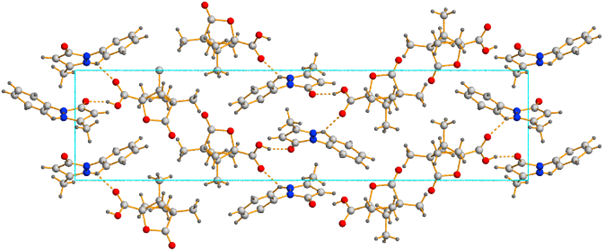
Fig. 1 Crystal structure of cocrystal Edaravon: Camphanic acid 2:1 (view along axis c)
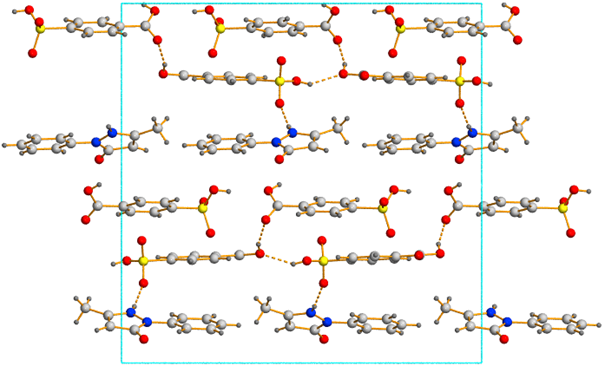
Fig. 2 Crystal structure of cocrystal Edaravon: 4- Sulfobenzoic acid 1:2 (view along axis c)
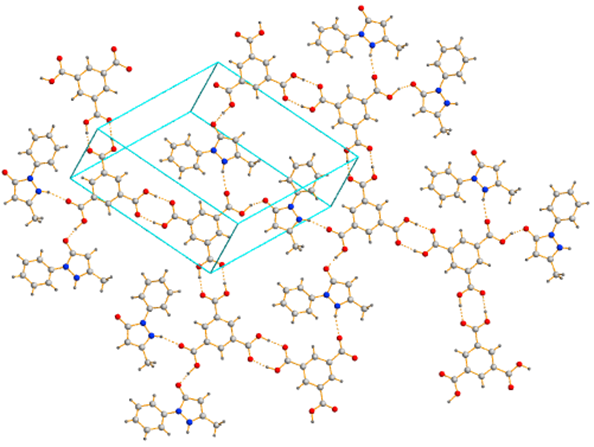
Fig. 3 Supramolecular 2D framework of continuum salt- cocrystal Edaravon: Trimesic acid 1:2
Table Crystallographic data
|
|
Edaravon: Camphanic acid 2:1 |
Edaravon: |
Edaravon: Trimesic acid |
|
Chemical formula |
C20H24N2O5 |
C24H22N2O11S2 |
C76H64N8O28 |
|
Mr |
372.41 |
578.56 |
1537.35 |
|
Cell setting Space group |
Orthorhombic |
Orthorhombic |
Triclinic |
|
T (K) |
293(1) |
293(1) |
293(1) |
|
a (Å) |
32.9448(10) |
19.911(3) |
7.5440(3) |
|
b (Å) |
7.94775(16) |
19.893(3) |
14.9690(12) |
|
c (Å) |
7.31362(18) |
6.5273(9) |
16.1120(7) |
|
α (°) |
90 |
90 |
81.442(5) |
|
β (°) |
90 |
90 |
78.327(4) |
|
γ (°) |
90 |
90 |
87.653(5) |
|
V (Å3) |
1914.98(8) |
2585.4(7) |
1761.89(18) |
|
Z |
4 |
4 |
1 |
|
R |
0.0538 |
0.1169 |
0.0478 |
1. M. Veverka, T. Dubaj, J. Gallovič, E. Švajdlenka, B. Meľuchová, V. Jorík, P. Šimon. Edaravone cocrystals: synthesis, screening, and preliminary characterization. Monatshefte für Chemie-Chemical Monthly, 2013, 144.9: 1335-1349.
2. S. L. Childs, G. P. Stahly, A. Park. The salt− cocrystal continuum: the influence of crystal structure on ionization state. Molecular pharmaceutics , 2007, 4.3: 323-338
3. A. N. Queiroz, et al. Tautomerism and radical-scavenging activity of edaravone by DFT methods. Journal of Computational and Theoretical Nanoscience, 2010, 7.1: 153-156.
4. T. Watanabe, M. Tahara, S. Todo. The novel antioxidant edaravone: from bench to bedside. Cardiovascular therapeutics, 2008, 26.2: 101-114.