Crystal structures of heterospin complexes based on Ni(II) and TCNQ
M. Hegedüs1, J. Černák1, J. Kuchár1, L. R. Falvello2
1Department of Inorganic Chemistry, Institute of Chemistry, P. J. Šafárik University in Košice, 041 54 Košice, Slovakia.
2Instituto de Ciencia de Materiales de Aragón (ICMA), Departamento de Química Inorgánica, University of Zaragoza–CSIC, E-50009 Zaragoza, Spain.
michal.hegedus@gmail.com
Complexes based on TCNQ (7,7’,8,8’-tetracyanoquinodimethane) anion-radicals as
carriers of unpaired electrons represent an extensively studied group of
materials. Due to the ability of TCNQ•- to directly coordinate to metal
atoms and, in addition, to form supramolecular chains via hydrogen bonding and π–π
interactions, this species offers a great deal of possibilities for crystal
engineering [1, 2].
The present study shows crystal structures of three nickel(II)
complexes with 2,2’-bipyridine (bpy) and its derivate
4,4’-dimethyl-2,2’-bipyridine (4,4’-dmbpy) containing TCNQ•-
in various forms. All three compounds were prepared by the reactions of hot
methanolic/ethanolic solutions of Ni(II) salt with bpy/4,4’-dmbpy and LiTCNQ, followed by
crystallization.
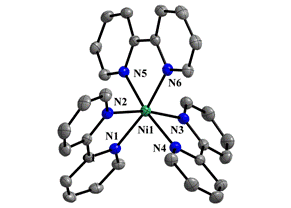
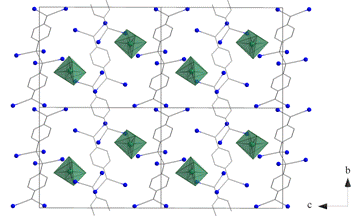
The crystal structure of [Ni(bpy)3]2(TCNQ−TCNQ)(TCNQ)2∙6H2O (1) was found to be composed of bulky complex cations [Ni(bpy)3]2+, uncoordinated TCNQ•- anion-radicals, uncoordinated σ-dimerized (TCNQ−TCNQ)2- dianions and three crystallographically independent, uncoordinated water molecules which are strongly disordered (Fig. 1 and Fig. 2). The nickel(II) atom within the [Ni(bpy)3]2+ complex cation is hexacoordinated by three bidentate aromatic ligands (Fig. 1). The Ni−N bond lengths range from
2.078(2) to 2.109(2) Å which are in the usual range of values. The σ-dimerized dianion (TCNQ−TCNQ)2- with a central C−C bond length of 1.653(11) Å is disordered with a pair of anion-radicals overlapped by their exo groups. The occupation ratio for disordered anion-radicals was refined to 0.753(9):0.247(9). Both forms of TCNQ, the anion-radicals and the σ-dimerized dianions, form supramolecular chains via π–π stacking of their external C(C≡N)2 groups. These chains alternate in the structure with layers of [Ni(bpy)3]2+ complex cations. The water solvate molecules along with the TCNQ•- anion-radicals form a three-dimensional supramolecular structure via O-H···O and O-H···N hydrogen bonds. The ionic crystal structures of two other compounds containing the 4,4’-dmbpy ligand will be discussed during the oral presentation.
1. L. Ballester, A. Gutiérrez, M. F. Perpiñán. Inorg. Chem., 36, (1997), 6390.
2. H. J. Choi, M. P. Suh. Inorg. Chem., 42, (2003), 1151.
|
|
|
|
Figure 1. Coordination environment of nickel(II) atom in [Ni(bpy)3]2+ |
Figure 2. Supramolecular chains of TCNQ with [Ni(bpy)3]2+ cations |