Some new findings about the polytypism of the mineral cronstedtite
J. Hybler1, J. Sejkora2, M. Števko3
1Institute of Physics, Academy of Sciences of the Czech Republic, Na Slovance 2,
CZ-182 21 Praha 8, Czech Republic
2Department of Mineralogy and Petrology, National Museum, Cirkusová 1740, CZ-193 00 Praha 9, Czech Republic
3 Department of Mineralogy and Petrology, Faculty of Natural Sciences, Comenius University, Ilkovičova 6, SK-84215 Bratislava, Slovakia
hybler@fzu.cz
Keywords: cronstedtite; 1:1 layer silicate; MDO polytypes 3T, 1M, 2H1, 2H2, 2M1; Non-MDO polytype 6T2; twinning.
Introduction
The rare mineral cronstedtite has been first described as early as in 1820 from the Vojtěch Mine in Příbram by Steinmann in 1820 [1, 2]. It is named in honor of Swedish chemist and mineralogist Axel Fredrik Cronstedt (*23.12.1722, †19.8.1765), one of founders of the modern mineralogy. Besides other things, he discovered mineral scheelite, element nickel, named element tungsten and defined zeolites.
In our country, cronstedtite has been later described from Kutná Hora by Vrba in 1886 [3, 4], Kaňk near Kutná Hora in 1957 [5], Litošice and Sovolusky [6, 7], Chvaletice [8, 7], Chyňava [9, 10] and quite recently from Pohled quarry near Havlíčkův Brod [11]. In Slovakia cronstedtite was found in Rožňava [12], and Nižná Slaná [13].
Other known localities are Gernrode, Lutherstadt Eisleben (Germany), Lostwithiel, Wheal Maudlin, Wheal Jane (Cornwall, UK), Herja, Chiuzbaia (Romania).
Cronstedtite typically occurs in low- and medium tempered hydrothermal veins with pyrite – on the surface, in cavities, inter-grown, or embedded in the polycrystalline mass or weathering products. Other accompanying minerals are: quartz, calcite, sometimes siderite, ankerite, galena, sphalerite, rhodochrosite, and/or rhodonite. It has been also found in metamorphic deposits [14-16], and in some meteorites – CM chondrites [17-23]. Its occurrence is assumed on some asteroids, e.g. Ceres.
The only synthesis of cronstedtite to date was referred by Pignatelli et al. [24].
Cronstedtite is black, with vitreous lustre and perfect cleavage along basal planes. Crystal habits are very variable: Triangular pyramids, truncated pyramids, triangular, hexangular, regular plates, laths, acicular, conical, sheaf like.
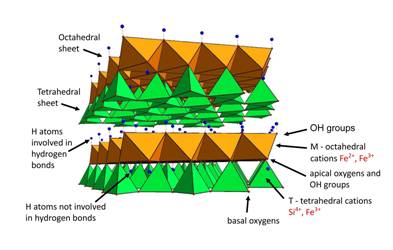
Frondel [25], and later Steadman & Nuttall [26, 27], and Steadman [28], revealed that cronstedtite belonged to 1:1 phyllosilicates of the serpentine-kaolinite group Its formula reads: (Fe2+3-x Fe3+x)(Si2-xFe3+x)O5(OH)4, (where x is usually in the range 0.5 to 0.8). Its structure is composed of edge-sharing octahedral and adjacent corner-sharing tetrahedral sheets, forming together 1:1 layer [29, 30]. Octahedral positions are occupied by Fe2+ and Fe3+, while in tetrahedral positions Si4+ is partially substituted for Fe3+. The proportion of Fe3+ in octahedra balances deficiency of charge in tetrahedra. Layers are interconnected by hydrogen bonds, with OH groups of octahedral sheets as donors and basal oxygen atoms of tetrahedral sheets as acceptors. (Figure 1)
Figure 1. Structure of cronstedtite, two 1:1 layers, side view.
The 1:1 layer silicates including cronstedtite are typical representatives of OD structure of layers [31, 32, 33, 34]. The polytypes of this OD family can be subdivided according to shifts and/or rotations of consecutive (identical) layers, into four OD-subfamilies, or Bailey’s [29, 30] groups: A (polytypes 1M, 2M1, 3T), B (2O, 2M2, 6H), C (1T, 3R, 2T), D (2H1, 6R, 2H2). The stacking rules are represented by following operations: ± ai/3 shifts for subfamily A, ± ai/3 shifts combined with 180º rotation for subfamily B, ± b/3 or no shift for subfamily C, ± b/3 or no shift combined with 180º rotation for subfamily D, where ai, b correspond to the vectors of trigonal (a~5.5, c~7.1 Å), and orthohexagonal protocells, respectively. Possible shift vectors together with the standard nomenclature used for descriptions of stacking sequences are presented in Figure 2. All polytypes listed above are so-called MDO (Maximum Degree of Order), whose stacking sequences contains the least possible kinds of equivalent triplets, in these cases only one.
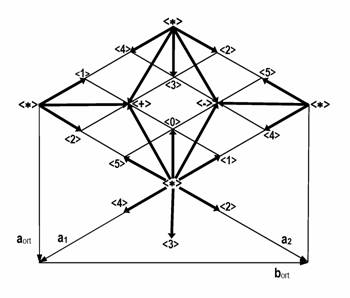
Figure 2. Possible displacement vectors of 1:1 layers with hexagonal and orthohexagonal cells indicated.
Recently structures of several most frequently occuring MDO polytypes were refined: 3T [35]; 1T [36]; 2H2 [37].
Experimental techniques
Subfamilies and polytypes are identified with aid of characteristic single crystal X-ray diffraction pattern of reciprocal lattice planes -2hhl/ hhl/ -h2hl and h0l/0kl/ -hhl, respectively. Traditionally these patterns were recorded by the precession method, but now-a-days they can be more quickly obtained with aid of modern diffractometers with area detectors during a user-defined pre-experiment [11]. This technique allows checking of many crystal fragments in a reasonable time. The precession-like images are obtained with aid of the diffractometer software [38]. The distributions of reflections along -21l/11l/-12l and 10l/01l/-11l reciprocal lattice rows were compared with graphical diagrams serving for the subfamily and polytype determination, respectively. Diagrams were published e.g. in [39, 40].
Some already checked crystals were later placed into resin, and polished sections for EMPA (electron microprobe analysis) studies were prepared. Selected good quality crystals were later used for data collection for the complete structure analysis.
For small crystals (of few micrometer size), the SAED (selected area electron diffraction) and EDT (electron diffraction tomography) was used. The powder diffraction is not reliable in many cases for the polytype determination [40].
Recent findings
Revisions of some older samples, EDT studies of synthetic material [24], and X-ray diffraction studies of samples from new occurrences (mainly of Pohled and Nižná Slaná [11, 13], see Figures 3, 4) were performed in last years. Some interesting results are summarized here.

Figure 3. Cronstedtite crystal in pyrite from Pohled, photo J. Sejkora.
Figure 4. SEM image of cronstedtite crystals from Nižná Slaná, photo M. Števko.
The rare 1M polytype.
This polytype (a = 5.5033(3), b = 9.5289(6), c = 7.3328(5) Å, β = 104.493(7)º, space group Cm) belongs to the group A, similarly as the much more abundant 3T (a = 5.499(2), c = 21.260(8) Å, space group P31). Although it is otherwise very rare in natural samples, it was found out as the dominant polytype in the cronstedtite synthetized by Pignatelli et al. [24]. Because of small size of crystals, the studies were performed with aid of SAED and EDT.
A rare cronstedtite-1M single crystal from Lutherstadt Eisleben, Germany was identified by Mikloš [41]. Despite a high degree of stacking disorder, it was used for the data collection and successful structure refinement [42].
Mixed crystals 3T+1M
Both polytypes belong to the subfamily A. The diffraction pattern is a superposition of patterns both polytypes. The 10l, 01l, -11l characteristic reflections of the 3T polytype reveal triple periodicity with respect of the 00l row. The presence of 1M polytype causes a change of intensities: every third reflections along the rows are stronger. In the plane perpendicular to the mirror plane of 1M, the l=3n reflections of 3T become stronger, while in other two planes the l=3n+1 or l=3n+2 reflections (of 3T) become stronger. Ďurovič [40] modelled diffraction patterns for various proportions of both polytypes.
Mixed crystals are very abundant in samples from Nižná Slaná and quite common in Pohled. Rarely they occur also in synthetic samples. The 1M polytype occurs preferentially in mixed crystals, rather than isolated.
Non-MDO polytype 6T2
This polytype was discovered for the first time in cronstedtite from Pohled [11], and it was relatively abundant in the material from this locality. The lattice parameters are a = 5.4976(3), c = 42.601(1) Å, space group P31. While the subfamily reflections correspond well to the subfamily A, the 10l, 01l, -11l characteristic reflections reveal sextuple periodicity. This pattern does not fit to any known MDO polytype. The structure model was found and refined by Hybler [43]. The stacking sequence contains two kinds of equivalent layer triplets, while the A subfamily allows construction of MDO polytypes using one kind of triplets only.
Since it has been already described another sextuple non-MDO polytype 6T1 (belonging to the subfamily D), of the isostructural mineral lizardite [44], the proposed Ramsdell symbol of the new polytype is 6T2.
The 2M1 polytype
This even more rare polytype (a = 5.497(2), b = 9.507(2), c = 14.267(6) Å, β = 97.25(3), space group Cc) of the subfamily A was detected in mixed crystals with 6T2 polytype in several specimens from Pohled [11]. It was also detected inter-grown with 3T and in separated crystals in the synthetic material [24]. Individual crystals of the size and quality appropriate for data collections were not found to date.
Twins by reticular merohedry of polytypes of the subfamily A
Reciprocal lattice sections of some crystals of the 3T polytype revealed existence of a twin by reticular merohedry of order 3. The twofold (or more general (2n-1)×60º) rotation parallel to the threefold axis of the R lattice exchanges obverse/reverse settings of the rhombohedral subfamily A structure. In the diffraction pattern subfamily reflections produced by both twin domains are superimposed. On the other hand, the characteristic reflections seem to be unaffected. Such a twinning is possible in the subfamily A.
This twinning appeared mostly in cronstedite-3T from Nižná Slaná [13], (about one half of crystals studied). Rarely it was indicated in one 6T2 and one 3T+1M mixed crystal from Pohled [11]. These studies provided evidence of this kind of twinning by X-ray diffraction. However, in the early study of Vrba [3], a picture of two interpenetrating trigonal pyramids rotated by 180º is presented. It seems probable, that this crystal is twinned by the law described above.
2H1+2H2 mixed crystals
Both polytypes belong to the subfamily D, characterized by regular alternation of 180º rotation of consecutive layers. In the 2H2 these rotations are combined with regular alternation of + b/3 and -b/3 shifts, while in the 2H1 polytype there is no shift of layers. Thus the crystals have tendency to “switch” between both polytypes during the growth. Lattice parameters of both polytypes are a = 5.5002(4), c = 14.195(1) Å, space groups: P63cm (2H1), P63 (2H2). The presence of 2H2 polytype is indicated by the occurrence of 10l, 01l, -11l characteristic reflections with l=2n, forbidden in P63cm, but allowed in P63 groups. The stronger these reflections are, the higher amount of 2H2 the crystal contains.
Mixed 2H1+2H2 crystals were found in the Pohled samples, most of them were 2H1 dominant, some of them were “almost pure” 2H1. The 2H2 dominant crystals were less frequent. In Nižná Slaná a rare higly disordered 2H1 crystal with traces of 2H2 was found.
Unknown polytype of the subfamily D
Some 2H1+2H2 mixed crystals from Pohled contained few extra reflections indicating a presence of further yet unknown polytype, probably of sextuple periodicity, not identical with the MDO polytype 6R. Because of a scarcity of reflections it could not be identified.
Chemical composition
Some selected crystals from Pohled and Nižná Slaná were studied by EMPA (electrom microprobe analysis). Results provided composition within range of the general formula. Small amounts of Cl were indicated in samples from both localities (0.009 to 0.06 apfu). Moreover, Nižná Slaná cronstedtite contained also an accessory amount of S (up to 0.11 apfu) [11, 13].
Further studies
Now-a-days, samples from the occurrence near Chyňava, Chvaletice, and from Litošice-Sovolusky-Morašice region are under investigation. In Chyňava, cronstedtite was encountered in a bore-hole in a depth 202 m [9, 10]. Near Litošice, a system of parallel veinlets of cronstedtite is developed in relatively large area (few km2). Almost identically looking samples were collected in shafts several kilometers apart.
1. J. J. Steinmann, Abhandlungen der Königlichen Böhmischen Gesselschaft der Wissenschaften Bd. 7, (1820).
2. J. J. Steinmann, Journal für Chemie und Physik, 32, (1821), 69.
3. K. Vrba, Sitzungsberichte der Königlichen Böhmischen Gesselschaft der Wissenschaften No. 3, (1886), 1.
4. K. Vrba, Věstník III. sjezdu českých přírodozpytců a lékařů 131. (1901), (in Czech).
5. F. Novák a kol., Sborník k osmdesátinám akademika F. Slavíka. Nakl. ČSAV, Praha, (1957), 315 (in Czech).
6. F. Novák & V. Hoffman, Rozpravy ČSAV, Řada Matematicko Přírodních Věd, 66, (1956), 31 (in Czech with an English abstract).
7. J. Hybler, Ceramics-Silikáty 42, (1998), 130.
8. F. Novák & J. Jansa, Čas. Min. Geol., 10, No.1, (1965), 75 (in Czech).
9. F. Fiala, Sborník Národního Muzea v Praze (Acta Musei Nationalis Pragae) řada B – Přírodní vědy, Vol. VII, (1951), No. 4.
10. F. Fiala & J. Kouřimský, Sborník Národního Muzea v Praze (Acta Musei Nationalis Pragae) 36B, (1980), 35.
11. J. Hybler, J. Sejkora, V. Venclík, Eur. J. Mineral., (2016), DOI: 10.1127/ejm/2016/0028-2532
12. C. Varček, J. M. Vasconselos, R. Petrová, P. Fejdi, Mineralia Slovaca 22, (1990), 565.
13. J. Hybler, M. Števko, J. Sejkora, Accepted for publication in: Eur. J. Mineral. (2016).
14. J. A. López García, J. I. Manteca, A. C. Prieto, B. Calvo, Boletín de la Sociedad Española de Mineralogía, 15-1, (1992), 21.
15. M. J. Gole, Can. Mineral., 18, (1980), 205.
16. M. J. Gole, Am. Mineral., 65, (1980), 8.
17. W. F. Müller, G. Kurat, A. Kracher, Tscherm. Min. Petr. Mitt., 26, (1979), 293.
18. K. Tomeoka & P. R. Buseck, Geochim. Cosmochim. Acta 49, (1985), 2149.
19. D. S. Lauretta, X. Hua, P. R. Buseck, Geochim. Cosmochim. Acta, 64, (2000), 3263.
20. T. J. Zega, P. R. Buseck, Geochim. Cosmochim. Acta, 67, (2003), 1711.
21. M. Schulte & E. Schock, Meteoritic and Planetary Science, 39, (2004), 1577.
22. M. Miyahara, S. Uehara, E. Ohtani, T. Nagase, M. Nishijima, Z. Vashaei, R. Kitagawa, Lunar Planetary Science, XXXIX, (2008), 199.
23. K. A. Dyl, C. E. Manning, E. D. Young, The implication of the cronstedite in water-rich planetesimals and asteroids. Astrobiology Science Conference 2010, League City, Texas (2010).
24. I. Pignatelli, E. Mugnaioli, J. Hybler, R. Mosser-Ruck, M. Cathelineau, N. Michau, Clay. Clay Miner., 61, (2013), 277.
25. C. Frondel, Am. Mineral., 47, (1962), 781.
26. R. Steadman & P. M. Nuttall, Acta Cryst, 16, (1963), 1.
27. R. Steadman & P. M. Nuttall, Acta Cryst, 17, (1964), 404.
28. R. Steadman, Acta Cryst. 17, (1964), 924.
29. S.W. Bailey, Clay. Clay Miner., 17, (1969), 355.
30. S.W. Bailey, Polytypism of 1:1 layer silicates. Hydrous Phyllosilicates (Exclusive of micas), Reviews in Mineralogy, Vol. 19, S.W. Bailey (editor), Mineralogical society of America, Washington, D.C., (1988), 9.
31. K. Dornberger-Schiff & S. Ďurovič, Clay. Clay Miner., 23, (1975), 219.
32. K. Dornberger-Schiff & S. Ďurovič, Clay. Clay Miner., 23, (1975), 231.
33. S. Ďurovič, Fortschr. Miner., 59, (1981), 191.
34. S. Ďurovič, Layer stacking in general polytypic structures. In: International Tables for Crystallography, Vol. C, 5-th ed., (2002), Section 9.2. Dordrecht: Kluwer Academic Publishers.
35. L. Smrčok, S. Ďurovič, V. Petříček, Z. Weiss, Clay. Clay Miner., 42, (1994), 544.
36. J. Hybler, V. Petříček, S. Ďurovič, L. Smrčok, Clay. Clay Miner., 48, (2000), 331.
37. J. Hybler, V. Petříček, J. Fábry, S. Ďurovič, Clay. Clay Miner., 50, (2002), 601.
38. Rigaku Oxford Diffraction, CrysAlis Pro, Data collection and data reduction GUI. Version 171.38.41q (2015).
39. Z. Weiss, & M. Kužvart, Clay minerals, their nanostructure and use. Charles University, Karolinum publishing house, Prague, (2005), 281 pp. (in Czech).
40. S. Ďurovič, Ceramics-Silikáty, 41, (1997), 98.
41. D. Mikloš, Symmetry and polytypism of trioctahedral kaolin-type minerals. Ph.D. thesis. Institute of Inorganic Chemistry, Slovak Academy of Sciences, Bratislava, Slovakia, (1975), 144 pp. (in Slovak).
42. J. Hybler, Acta Cryst., B70, (2014), 963.
43. J. Hybler, Eur. J. Mineral., (2016), DOI: 10.1127/ejm/2016/0028-2541
44. S. H. Hall, S. Guggenheim, P. Moore, S.W. Bailey, Can. Mineral. 14, (1976), 314.
The study was supported by the grant 15-04204S of the Czech Science Foundation.