Synthesis, characterization and crystal structures of two polymorphs of [Co2(o-van-en)3]·4CH3CN
A. Vráblová1, J. Černák1, L. Falvello2, M. Tomás2
1Department of Inorganic Chemistry, Institute of Chemistry, P. J. Šafárik University in Košice, Moyzesova 11, 041 54 Košice, Slovakia
2Facultad de Ciencias, Universidad de Zaragoza, Pedro Cerbuna 12, 50009 Zaragoza, Spain
anna.vrablova@student.upjs.sk
Schiff
bases are often used as ligands for the coordination of cobalt due to their
multi-donor properties [1] and consequent versatility. In our quest for Co(II) complexes as magnetically
active materials [2; 3] we have isolated two polymorphs of [Co2(o-van-en)3]×4CH3CN (1 and 2) based
on the Schiff base ligand
o-van-en
synthesized by the reaction
of ethylenediamine with o-vanillin in 1:2 molar ratio (Scheme 1).
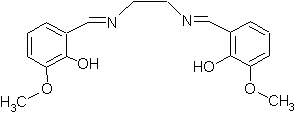
Scheme 1. Structural diagram of H2(o-van-en).
The triclinic (P-1) polymorph 1 was obtained after room temperature crystallization of the acetonitrile solution of the reaction product of the Schiff base with cobalt hydroxide in air. Differences in reaction and crystallization conditions produced the monoclinic form 2 (space group P21/c). Both polymorphs were chemically and spectroscopically characterized, and the results indicated spontaneous oxidation of Co(II) to Co(III). The crystal structures of both 1 and 2 are built up of molecules of the centrosymmetric dinuclear complex [Co2(o-van-en)3], in which each Co(III) atom is coordinated by one tetradentate o-van-en ligand in an uncommon bent fashion. The pseudooctahedral coordination of the Co(III) atom is completed by one phenolato O and one amidic N atom of the same arm of the bridging o-van-en ligand. In addition, the asymmetric units of both polymorphs contain two acetonitrile solvate molecules. In polymorph 2, consistent with the symmetry of the space group, the dinuclear {Co2} units are arranged in an alternating ABABAB fashion, in contrast to the AAA arrangement of the dinuclear units in polymorph 1. As a consequence, both polymorphs differ in the positions of the acetonitrile solvate molecules and in the pattern of intermolecular interactions. Differences of some geometrical parameters, e.g., torsion angles were observed, too.
1. M. Andruh, Dalton Trans., 44, (2015), 16633-16653.
2. E. Burzurí, J. Campo, L. R. Falvello, E. Forcén-Vázquez, F. Luis, I. Mayoral, F. Palacio, C. Sáenz de Pipaón, M. Tomás, Chem. Eur. J., 17, (2011), 2818-2822.
3. L. Smolko, J. Černák, J. Kuchár, J. Miklovič, R. Boča, J. Mol. Structure, 1119, (2016), 437-441.
This work was supported by P. J. Šafárik University in Košice (VVGS-PF-2016-72623, VVGS-2016-265). The stay of AV in University of Zaragoza was supported by National Scholarship Programme of the Slovak Republic.