STRUCTURE ANALYSIS OF NANOFIBERS PREPARED BY NANOSPIDER TECHNOLOGY
P. Ryšánek1, P. Čapková1, M. Munzarová2, A. Čajka1, M. Barchuk1
1Faculty of Science, J. E. Purkyně University, České mládeže 8, 400 96 Ústí nad Labem
2Nanovia, s. r. o., Litvínov, Podkrušnohorská 271, 436 03 Litvínov – Chudeřín
Polymer nanofibers are used in wide range of activities in everyday life, such as: filtration [1], protective closing, pharmaceuticals [2], etc. The greatest attention is paid on nanofibers, that have great biocompatibility. These nanofibers can be used as wound covers in biomedicine. The first representative is nylon 6, which has very good biocompatibility and also very good mechanical properties [3]. The other representative is chitosan, which is polysacharide, and due to its origin it has also very good biocompatibility. Chitosan itself have also antibacterial properties [4, 5].
The structure of nylon 6 and chitosan was studied by many authors [6 - 9], but the structure of these nanofibers prepared by NANOSPIDER technology is less studied [10, 11], although this technology is widely used in industry and due to is the knowledge of the structure very important, because the structure has significant influence on nanofiber properties.
Nylon 6
Structure of nylon 6 polymer is very well known. It was resolved that nylon 6 is polymorphic and has two crystal structures: alpha form which was described by Brill [12] and Holmes [13] and gamma form which was determined by Holmes [13]. Both structures are monoclinic and differ from each other by density and arrangement of polymeric chains. The arrangement of the polymeric chains can be seen on Figure 1.
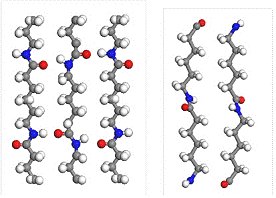
Figure 1: Arrangement of polymer chains of nylon 6 in ab plane in crystal structure of alpha phase (left) and gamma phase (right)
It is known that the electrospinning preparation of nylon 6 nanofibers leads to three structural phases: alpha, gamma and amorphous phase of nylon 6. The dependence of nylon 6 structure on the NANOSPIDER arrangement was invetsigated. It has been determined, that the phase composition of nylon 6 nanofibers (i.e. the content of alpha, gama and amorphous phase) depends on electrode distance. In our previous work the core-shell structure model has been suggested, based on combination of XRD and XPS measurements [10]. The fibers exhibit also very strong texture in direction (010). The typical XRD pattern of nylon 6 nanotextile is on Figure 2.
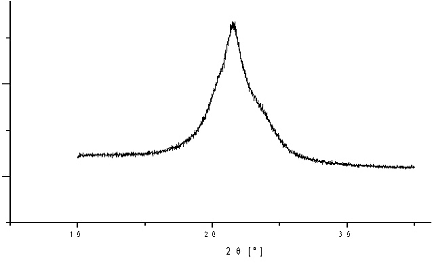
Figure 2: Typical XRD profile of nylon 6 nanotextile
Chitosan
It is difficult to prepare pure chitosan nanotextiles using electrospinning [8]. Therefore it is often used the mixture of chitosan with other polymers [14, 15]. For easier electrospinning we used mixture of chitosan and polyethylene oxide (PEO). For further applications in medicine the mixture of chitosan, PEO and gelatin has been used. The nanotextile was also cross-linked at 130°C. The research was aimed in order to investigate the effect of gelatin and cross-linking on the nanotextile structure and properties. It has been found, that:
- The presence of gelatin has strong effect on nanofiber structure, as it prevents the crystallization of chitosan.
- After the thermal crosslinking the gelatin climbs to surface of the fibers and reduces the porosity of the nano-textile.
- The thermal crosslinking itself (without gelatin) also disrupts the structure of nanofibers and reduces the crystallinity of chitosan and PEO.
- H. R. Ant, M. P. Bajgai, Ch. Yi, R. Nirmala, K. T. Nam, W. Baek, H. Y. Kim., Colloids and Sur faces A: Physicochemical and Enginering Aspects. 370 (2010) 87-94.
- P. Raghavan, X. Zhao, J. K. Kim, J. Manuel, G. S. Chauhan, J. H. Ahn, C. Nan, Electrochim. Acta 54 (2008), 228-234
- J. L. Pey, Anti_Corros Methods Matter 44, (1997), 94-99
- F-L. Mi, Y-C. Tan, H-F. Liang, H-S. Sung, Biomaterials 23 (2002), 181–191
- I. Leceta, P. Guerrero, I. Ibarburu, M.T. Duenas, K. de la Caba, J. Food Engineering 116 (2013), 889-899
- S. Zhang, W. S. Shim, J. Kim, Materials 30 (2009), 3659-3666
- S. S. A. Deyah, M. H. E. Newehy, R. Nimala, A. A. Megeed, H. Y. Kim, Korean Journal of Chemical Engineering 30 (2013), 422-428
- S.D. Vrieze, P. Westbroeak, T.V. Camp, L. van Langenhove, J. of Materials Sc. 42 (2007), 8029–8034
- K. Ohkawa, K-I. Minato, G. Kumagai, S. Hayashi, H. Yamamoto, Biomacromolecules 7 (2006), 3291–3294
- P. Čapková, A. Čajka, Z. Kolská, M. Kormunda, J. Pavlík, M. Munzarová, M. Dopita, D. Rafaja, J. Polym. Res. 22 (2015), 101
- M. Barchuk, P. Čapková, Z. Kolská, J. Matoušek, D. Poustka, L. Šplíchalová, O. Benada, M. Munzarová, J. Polym. Res. 23 (2016), 19-25
- R. Brill, Z. Physik. Chem B 53 (1943), 61-66
- R. Holmes, C. W. Burn, D. L. Smith, J. Polym. Sci. 17 (1955), 619-624
- M. Spasova, N. Manolova, D. Paneva, I. Rashkov, e-Polymers 56 (2004), 1–12
- K. Ohkawa, T. Kitagawa, H. Yamamoto, Macromolecular Materials and Engineering 289 (1) (2004), 33–40
The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2015073.