Interaction of nylon 6 with antibacterial molecules
Iryna Gren, Marek Malý, Pavla Čapková
Faculty of Science, Jan Evangelista Purkyně University in Ústí nad Labem, Česká mládeže 8, 40096 Ústí nad Labem
In connection with the development of technologies for production and use of nanomaterials, which are due to particular physical, chemical, biological, pharmacological and mechanical properties are able to cause unpredictable effects on biological objects in modern science there is a problem ethical use of nano substances and their risk assessment for the body humans and the environment. Although studies the effects of certain nanostructures performed very active, there are difficulties in forecasting migration and interaction of nanoparticles.
Nylon 6 is a linear addition polymer of caprolactam. This means, that the nylon 6 can be seen as the product of intramolecular interactions carboxyl group and 6 -amino-caproic acid [1,2]. Upon cooling nylon-6 from the melt, a semicrystalline structure is obtained, which is characterized by polymorphism. There are two crystalline phases α and γ (see figure 1.) [3,4,5]. They differ in arrangement of chains and network of hydrogen bonds. Interaction of nylon 6 with antibacterial molecules is interesting for the design of antibacterial nanofiber filters [6]. To model these interactions we used molecular modeling using empirical force field in Materials Studio modeling environment.
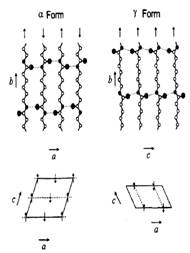
Figure 1. Structures of the α and γ forms of nylon-6
Three types of antibacterial molecules have been used in the present study: chlorhexidine, BTAB (benzyltrimethyl amonium bromide), DTAB (dodecyltrimethyl amonium bromide) [7]. Interaction energy, which defines the stability of the complex nylon6-antibacterial molecule has been calculated and compared for each individual antibacterial substance [8].
The preferable orientation of molecules on
the nylon6 surface, represented by crystalline phase, was studied for very low
and realistic surface density of all 3 antibacterial ligands.
The authors acknowledge the assistance provided by the Research Infrastructure NanoEnviCz, supported by the Ministry of Education, Youth and Sports of the Czech Republic under Project No. LM2015073.
1. Arimoto, H., Ishibashi, M., and Hirai, H., Crystal Structure of the α form of Nylon 6, J. Polym. Sci. A 3, 317-326 (1965).
2. Shenoy S.L, Bates W.D, Frisch H.L, Wnek G.E (2005), Role of chain entanglements on fiber formation during electrospinning of polymer solutions: good solvent, non-specific polymer-polymer interaction limit, Polymer 46:3372.
3. Siddharth Dasgupta, Wills B. Hammond, and William A.Goddard III, Crystal Structures and Properties of Nylon Polymers from Theory, Received december 22, 1994. Revised Manuscript Received July 19, 1996.
4. N.S. Murthy, R.G. Bray, S.T. Correale, R.A.F. Moore Drawing and annealing of nylon-6 fibres: studies of crystal growth, orientation of amorphous and crystalline domains and their influence on properties, Polymer, 36 (20) (1995), pp. 3863–3873.
5. Yi Liu, Li Cui, Fangxiao Guan, Yi Gao, Nyle E. Hedin, Lei Zhu,*, and Hao Fong† Crystalline Morphology and Polymorphic Phase Transitions in Electrospun Nylon 6 Nanofibers, Macromolecules. 2007 ; 40(17): 6283–6290.
6. Pavla Čapková*, Antonín Čajka, Zdenka Kolská, Martin Kormunda, Jaroslav Pavlík, Marcela Munzarová, Milan Dopita, David Rafaja, Phase composition and surface properties of nylon-6 nanofibers prepared by nanospider technology by various electrode distances, Article in journal of Polymer Research 22(6), June 2015.
7. Veggeland, Kirsti; Nilsson, Svante. Polymer-Surfactant Interactions Studied by Phase Behavior, GPC and NMR, Langmuir (1995), 11 (6), 1885-92.
8. Shailesh M. Kolhe,Ashok Kumar, Radiation-induced grafting of vinyl benzyl trimethyl ammonium chloride onto nylon-6 fabric, Radiation Processing Group, 14 July 2006.