Determination of phase composition of iron sinters by methods
of
X-ray diffraction
P. Vranec, M. Černík, A. Mašlejová
U. S. Steel Košice, s.r.o., Vstupný areál U. S. Steel, 044 54
Košice
pvranec@sk.uss.com, mcernik@sk.uss.com, amaslejova@sk.uss.com
The production of most pig iron is based on the use of iron sinter which consists of various mineral phases formed during the sintering process of iron ore, fluxes and coke ash. Chemical reactions at high temperatures take place during the sintering process. Particles of iron ore and flux interact with each other to form a sinter cake, which consist of iron ore, silico-ferrites of calcium and aluminium (SFCA, Silico-Ferrite of Calcium and Aluminium) [1, 2], dicalcium silicate and amorphous phase. The results of studies by Scarlett et al. [3] indicate that the iron sinter structure is formed by the following phases: iron oxides (ca. 40 – 70 vol%), ferrites (mainly SFCA, ca. 20 %), calcium silicates (up to ca. 10 %) and amorphous phase (up to ca. 10 %). Iron sinters can occasionally contain also phases such as sulphides (FeS), pyroxenes ((Mg,Fe)SiO3), quartz and lime. Summary of common mineral phases occurring in the iron sinters is given in Table 1.
Iron oxides are present in the iron sinters as a residues of non-reacted iron ores or as products crystallizing from the melt. The most common are hematite and magnetite; the presence of wüstite is observed only in the case of iron sinters produced with increased amount of fuel. Calcium ferrites are secondary phases which are formed by a reaction between Fe2O3 and CaO, which comes from the additives. The most common are dicalciumferrite (Ca2Fe2O5) and monocalciumferrite (CaFe2O4). Ferrites of complex composition (SFCA) are however highly represented in the iron sinters. Based on the high mutual chemical affinity of lime and SiO2, the presence of larnite (Ca2SiO4), as well as hedenbergite (CaFeSi2O6) and pseudowollastonite (CaSiO3) is also observed, which are however ineligible mineral phases due to their acidity. Dominant component of the amorphous phase in iron sinters is mainly SiO2.
In U. S. Steel Košice, s.r.o. the production of pig iron is also based on the use of iron sinter prepared at four sintering bands. Identification of phase composition of the industrial iron sinters plays a key role in optimization of the sintering process, as well as appropriate selection of the used raw materials. Therefore, the laboratory of X-ray diffraction at the Department of Metallography and Failure Analysis (MaFA) deals with the determination of the phase composition of iron sinters as well. Since the 2005 more than 400 samples have been measured and evaluated at the MaFA.
Diffraction patterns of iron sinters were measured on Bragg-Brentano goniometer equipped with line detector, which significantly shorten the measurement time. For better resolution of the diffraction pattern the Co Kα radiation with the voltage of 40 kV and current of 35 mA was used. Evaluation of the phase composition was carried out in TOPAS software from Bruker Company. The key factor at evaluation of phase composition from the measured diffraction patterns was the availability of the structure data, where until recently the diffraction patterns were evaluated without the presence of SFCA phases. These were replaced by other phases of similar composition, i.e. Ca4Fe9O17, CaFe5O7, Ca2Fe22O33, CaAl4O7 (Grossite), Na(AlSi3O8) (Albite) and others. Recently, the analyses of iron sinters include all available and necessary structure data, what increase the quality of the evaluation. Evaluated diffraction pattern of typical industrial iron sinter is given in Fig. 1 and general phase composition of the analyzed samples of iron sinters together with minimal, maximum and average content of each mineral phase is summarized in Table 2.
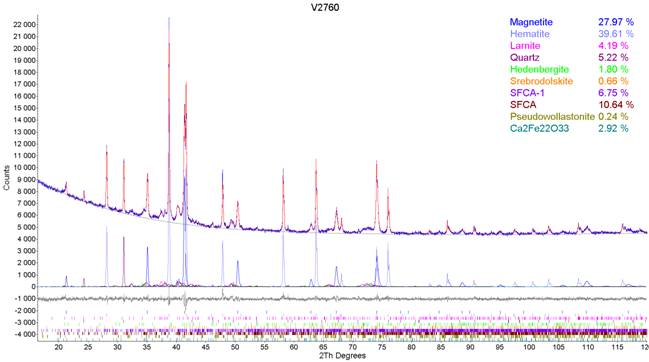
Figure 1. Sample 1, detail of the refined diffraction pattern.
Table 1. Properties of common phases present in iron sinters.
|
Group |
Mineral Phase |
Rational Formula |
Stechiometric Formula |
Lattice Type |
|
Iron oxides |
Hematite |
α-Fe2O3 |
α-Fe2O3 |
Trigonal |
|
Magnetite |
FeO·Fe2O3 |
Fe3O4 |
Cubic |
|
|
Calcium ferrites |
Calcium ferrite |
CaO·Fe2O3 |
CaFe2O4 |
Orthorhombic |
|
Calcium diferrite |
CaO·2Fe2O3 |
CaFe4O7 |
Monoclinic |
|
|
Dicalcium ferrite |
2CaO·Fe2O3 |
Ca2Fe2O5 |
Orthorhombic |
|
|
Brownmillerite |
2CaO·Al2O3·Fe2O3 |
Ca2(Al,Fe)2O5 |
Orthorhombic |
|
|
SFCA |
* |
* |
Triclinic |
|
|
Silicates |
Larnite |
β-2CaO·SiO2 |
β-Ca2SiO4 |
Monoclinic |
|
Hedenbergite |
CaO·FeO·2SiO2 |
CaFeSi2O6 |
Monoclinic |
|
|
Pseudowollastonite |
CaO·SiO2 |
CaSiO3 |
Monoclinic |
* Due to the variable composition, no official formula is accepted
Table 2. General phase composition of the analyzed iron sinter samples.
|
Identified phase composition |
Min |
Max |
Average |
||
|
Chemical Formula |
Mineralogical name |
Space Group |
Content [wt%] |
Content [wt%] |
Content [wt%] |
|
Fe3O4 |
Magnetite |
(227) Fd-3m |
14.5 |
46.6 |
28.9 |
|
Fe2O3 |
Hematite |
(167) R-3c |
10.2 |
56.3 |
32.5 |
|
CaSiO4 |
Larnite |
(014) P21/c |
2.5 |
8.3 |
5.6 |
|
SiO2 |
Quartz |
(152) P3121 |
2.7 |
7.8 |
4.7 |
|
CaFeSi2O6 |
Hedenbergite |
(015) C2/c |
1.4 |
4.7 |
2.8 |
|
Ca2Fe2O5 |
Srebrodolskite |
(062) Pnma |
0.2 |
4.0 |
0.8 |
|
Ca2.45Fe9.04Al1.74Fe0.16Si0.6O20 |
SFCA |
(002) P-1 |
6.1 |
19.7 |
11.9 |
|
Ca3.18Fe14.66Al1.34Fe0.82O28 |
SFCA-I |
(002) P-1 |
1.7 |
20.7 |
10.2 |
|
CaSiO3 |
Pseudowollastonite |
(002) P-1 |
0.1 |
0.8 |
0.4 |
|
Ca2Fe22O33 |
Calcium Iron Oxide |
(155) R32 |
0.0 |
4.4 |
2.3 |
1 J.D.G. Hamilton, B.F. Hoskins, W.G. Mumme, W.E. Borbidge, M.A. Montague: The crystal structure and crystal chemistry of Ca2.3Mg0.8Al1.5Si1.1Fe8.3O20 (SFCA): solid solution limits and selected phase relationship of SFCA in the SiO2–Fe2O3–CaO(–Al2O3) system, Neues Jahrbuch für Mineralogie, 161, 1-26 (1989).
2 W.G. Mumme, J.M.F. Clout, R.W. Gable: The crystal structure of SFCA-I, Ca3.18Fe3+14.66Al1.34Fe2+0.82O28, a homologue of the aenigmatite structure type, and new crystal structure refinements of β-CFF, Ca2.99Fe3+14.30Fe2+0.55O25 and Mg-free SFCA, Ca2.45Fe3+9.04Al1.74Fe2+0.16Si0.6O20, Neues Jahrbuch für Mineralogie, 173, 93-117 (1998).
3 N.V.Y. Scarlett, M.I. Pownceby, I.C. Madsen, A.N. Christensen: Reaction sequences in the formation of silico-ferrites of calcium and aluminum in iron ore sinter, Metallurgical and materials transactions B, 35B, 929-936 (2004).