Molecular simulations of interactions among CdS nanoparticles and montmorillonite
M. Pšenička, M. Pospíšil
Charles University in Prague, Faculty of Mathematics and Physics, Department of Chemical Physics and Optics, Ke Karlovu 3, 121 16 Prague 2, Czech Republic
milan.psenicka@matfyz.cz
CdS nanoparticles are semiconductors with a band gap about 2.5 eV and having photocatalytic properties for reduction of CO2. CdS occurs in two different crystal structures (i) as hexagonal greenockite structure and (ii) cubic hawleyite structure. Nanostructured II-IV semiconductors such as CdS possess properties that make them very photosensitive materials. It is well known that the most of the properties strongly depends on the size and shape of nanoparticle [1]. Preparation of CdS nanoparticles was done in [2] by reaction of cadmium acetate and sodium sulphide in presence of cetyltrimethylamonium bromide (CTAB). CTAB molecules were used to control the diameter of CdS nanoparticles and to preserve their photocatalytic properties [3].
Montmorillonite (MMT) is widely used clay mineral in many branches of industry for applications like the intercalation processes, sorption, fillers to polymers, photoluminiscence, catalysis and carriers [4]. In this case MMT was used as host matrix for adsorbed CdS nanoparticles on its surface.
Our work is focused on description of mutual arrangement of CdS nanoparticles with CTA+ alkylamonium chains and MMT as well as the calculation of interaction energies between each part of these components by molecular simulations methods. These methods are derived on the parameters obtained from experimental measurements and resultant calculated models and their properties are compared with experimental data to obtain the best agreement between them.
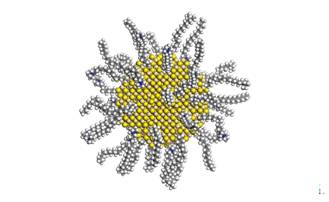
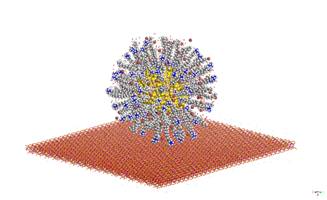
Nanocluster of CdS in the shape of sphere with radius 2 nm was created for both greenockite and hawleyite structure based on their crystallographic data. These nanoclusters were surrounded by various amounts of optimized CTA+(10-100, with step of 10 CTA+) in the form of monolayer and bilayer arrangement. The optimized alkylamonium chains of CTA+ were placed perpendicular to the surface of CdS nanoparticle. The geometry of micelles consisting of CdS nanoparticle and CTA+ for monolayer and bilayer arrangement without water molecules were optimized in Universal force field. The charges of CdS for both greenockite and hawleyite nanoparticles were calculated by the QEq method [5]. The optimized micelles were surrounded by water envelope in the form of sphere layer which consist of 1000 water molecules and then whole structure was placed above MMT surface and optimized.
Interaction energies between CdS nanoparticle, CTA+ , MMT surface and water were calculated for each optimized model. Comparison of sublimation energies for monolayer and bilayer arrangement of CTA+ molecules was done. Based on this comparison we suggested that for larger amount of CTA+ molecules (>30) bilayer arrangement is preferred. Obtained experimental and molecular simulation results confirmed that CTA+ molecules are able to effectively stabilize CdS nanoparticles forming colloidal dispersions and that the size of CdS nanoparticles can be controlled by the CTA+ concentration. Moreover comparison between CdS and ZnS nanoparticles [6] prepared by the same methods was done.
|
|
|
Figure 1. CdS nanoparticle with 40 CTA+ molecules in form of bilayer arrangement. |
Figure 2. CdS nanoparticle with 100 CTA+ molecules in form of bilayer deposited on MMT surface and surrounded by water envelope. |
1. Kotkata, M. F., Masoud, A. E., Mohamed, M. B., Mahmoud, E. A., Physica E: Low-Dimensional Systems and Nanostructures, 41(8), (2009), 1457–1465.
2. Praus, P., Kozák, O., Kočí, K., Panáček, A., Dvorský, R., Journal of Colloid and Interface Science, 360(2), (2011), 574–9.
3. Curri, M. L., Agostiano, A., Manna, L.,Monica, M. D., Catalano, M., Chiavarone, L., J. Phys. Chem. B, 104, (2000), 8391–8397.
4. Bergaya F., Handbook of clay science. Amsterdam: Elsevier, 2013.
5. Rappé, K. A., Goddard, A. W., J. Phys. Chem., 95, (1991), 3358-3363.
6. Praus, P., Dvorský, R., Horínková, P., Pospíšil, M., Kovář, P., Journal of Colloid and Interface Science, 377(1), (2012), 58–63.