X-Ray and NMR Study of Protonation State of Pharmaceutical Amides
Eliška Skořepová1, Michal Hušák1, Luděk Ridvan2, Marcela Tkadlecová2
1Department of Solid
State Chemistry, Institute of Chemical Technology Prague, Technicka 5, Prague 6,
Czech Republic
2Solid State Development, Zentiva k.s., U Kabelovny 130, Prague 10,
Czech Republic
The search for new solid forms of an active pharmaceutical ingredient (API) is an important step in a drug development. Salts and co-crystal are multicomponent solids but in different protonation (ionization) states. In salts, there is a proton transfer between the molecular components, making it contain cations and anions. On the other hand, co-crystals are made up from neutral molecules held together by non-bonded interactions. Often, an API has a low water solubility, which then leads to a low oral bioavailability. This physico-chemical problem can be solved by a co-crystal or salt formation. An API with one such problem is agomelatine, a melatonergic antidepressant. However, agomelatine is an amidic compound and, since amides are generally considered as neutral (non-ionisable), it was quite a surprise, when agomelatine, in the combination with certain acids, produced salts. Several co-crystals were prepared as well. The novel crystalline phases were prepared either by a slow evaporation or precipitation of the solution of agomelatine with the respective acid. The prepared solid phases were analyzed by powder X-ray diffraction and the structures were solved from single-crystal or powder X-ray diffraction data. The crystal structures of six agomelatine salts (with hydrobromic, hydroiodic, triiodic, sulfuric, methansulfonic and benzensulfonic acids) and of five co-crystals (with citric acid, maleic acid, oxalic acid, 4-hydroxybenzoic acid and hydroquinone) were solved. In addition to the anhydrous forms, in the case of three of the salts (with hydroiodic, sulfuric and methansulfonic acids), the structures of hydrated/solvated forms were described as well. In all the salts, the agomelatine molecule was positively charged. Specifically, the amide oxygen was protonated. The proton transfer and the salt formation were also confirmed by solid state NMR and the ΔpKA calculation. The comparison between single-crystal X-ray diffraction, solid state NMR and ΔpKA data revealed a strong correlation, meaning that all three methods can be reliably used to distinguish between salts and co-crystals. For pharmaceuticals, the determination whether the material is a salt or a co-crystal is interesting not only academically, but also from the regulatory point of view. Therefore, our findings may play a crucial role in the future development of the multicomponent solid phases of agomelatine and other amidic pharmaceutical compounds.
This work was supported by the Grant Agency of Czech Republic, Grant no. 106/14/03636S and received financial support from specific university research (MSMT No 20/2015).
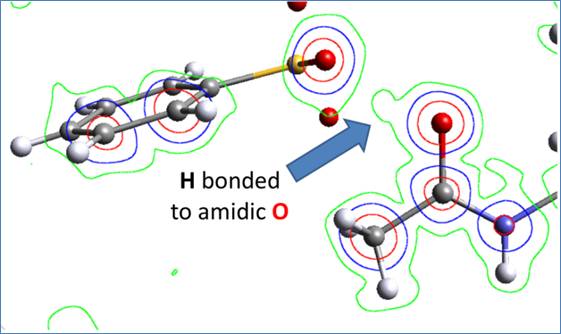
A slant Fourier map of the observed electron density and the molecular model of agomelatine – benzensulfonic acid (a proton transfer in a salt).