A self-assembly of copper(II) carboxylate through H-bonds into supramolecular structure and supramolecular networks
J. Moncoľ
Institute of Inorganic Chemistry, Faculty of Chemical and
Food Technology,
Slovak University of Technology in Bratislava, SK-81237 Bratislava, Slovakia
Email: jan.moncol@stuba.sk
Coordination compounds connecting through hydrogen bonds are used as building block for construction of supramolecular networks. Some copper(II) carboxylate complexes have shown that the intermolecular H-bonds can modified their magnetic properties. We have recently published mononucler molecular complex,1 binuclear molecular complex2 and more coordination polymers3,4 which exhibit similar magnetic properties. Very similar magnetic properties of mononuclear, binuclear as well as polymeric complexes could be explained by the presence of very similar H-bond supramolecular synthons that are pathway for antiferromagnetic interactions.
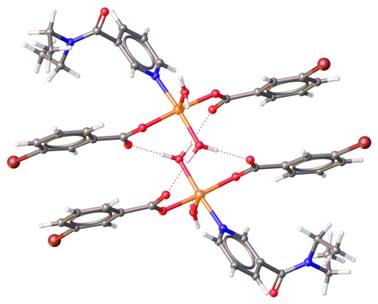
The lecture will present new supramolecular dimers [Cu(3-NO2bz)2(ina)(H2O)2].H2O, [Cu(3-NO2bz)2(ina)(H2O)2].2H2O, [Cu(3,5-Cl2bz)2(H2O)3], [Cu(3-Brbz)2(dena)(H2O)2], [Cu(3-Brbz)2(dena)(H2O)2] (Figure 1), (3-NO2bz = 3-bromobenzoate, 3-Brbz = 3-bromobenzoate, 3,5-Cl2bz = 3,5-dichlorobenzoate, dena = N,N-diethylnicotinamide, ina = isonicotinamide) and series of 1D-coordination polymers [Cu(3-Clbz)2(μ-dena)(H2O)]n (Figure 2), [Cu(4-Clbz)2(μ-dena)(H2O)]n, [Cu(3,5-Cl2bz)2(μ-dena)(H2O)]n (3-Clbz = 3-chlorobenzoate, 4-Clbz = 4-chlorobenzoate, 3,5-Cl2bz = 3,5-dichlorobenzoate, and dena as bridging ligand) with similar system of hydrogen bonds and properties. Electronic structure from multipole refinement of polymeric complex [Cu(4-Clbz)2(μ-dena)(H2O)]n will be also presented.
The hydrogen bonds described by R22(10) and R22(12) supramolecular synthons, formed by coordinated water molecule and two carboxylic group on each Cu2+ ions could create supramolecular dimer of two mononuclear complex molecules or 2D-supramolecular layers of 1D-coordination polymers.
1. Z. Vaskova, J. Moncol, M. Korabik, D. Valigura, J. Svorec, T. Lis, M. Valko, M. Melnik, Polyhedron, 29, (2010), 154.
2. D. Valigura, J. Moncol, Z. Pucekova, T. Lis, J. Mrozinski, M. Melnik, Eur. J. Inorg. Chem., (2006), 3813.
3. M. Korabik, Z. Repicka, L. Martiska, J. Moncol, J. Svorec, Z. Padelkova, T. Lis, M. Mazur, D. Valigura, Z. Anorg. Allg. Chem., 637, (2011), 224.
4. Z. Vaskova,J. Moncol, M. Korabik, J. Medvecka, J. Svorec, Z. Padelkova, M. Valko, D. Valigura, Polyhedron, 30, (2011), 86.
 .
.
Figure 1 Structure of supramolecular dimmer of [Cu(3-Brbz)2(dena)(H2O)2].
.
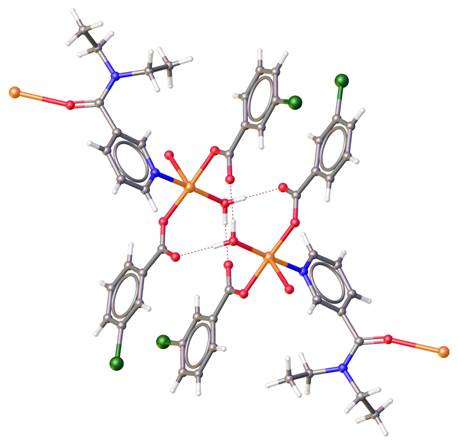
Figure 2. Structure of polymeric complex [Cu(3-Clbz)2(μ-dena)(H2O)]n..