Polymorphs of arsenic sulfide and their promising anti-cancer effects
A. Zorkovská1, Z. Bujňáková1,
P. Baláž1, J. Sedlák2
1Institute
of Geotechnics,
2Cancer
Research Institute,
zorkovska@saske.sk
Keywords: realgar, pararealgar, milling, nanoparticles, anti-cancer effect
Abstract
Nanosuspensions of arsenic sulfide (As4S4) polymorphs (realgar and pararealgar) were prepared by circulation mill, with average particle size below 150 nm. The nanosuspensions were stable up to six weeks. Their anti-cancer effects were tested and compared on human lung cancer H460 cell line. Induction of DNA damage and increase of apoptotic cells was observed. The arsenic dissolution from the nanosuspensions in simulated gastric and intestinal fluids reached 12–13.5%.
Introduction
Arsenic
sulfides have been utilized for a long time in the manufacture of cosmetics,
foods, glass, insecticides, pigments, and in medicine as well [1]. In Western
medicine, approximately 60 different arsenic preparations have been developed
and used in pharmacological history. In traditional Chinese medicines different
forms of mineral arsenicals are used, and realgar
alone is included in 22 oral remedies, recognized by the Chinese Pharmacopeia
Committee (2005). In the recent years its potential anticancer effects have
been studied [2,3]. Production of nanocrystals
is an approach to increase the drug solubility and its bioavailability. Here,
the arsenic sulfides were prepared as nanosuspensions
in circulation mill.
The As4S4 has at least three distinct polymorphs: i) the α-As4S4
phase, with monoclinic crystal structure (space group P21/n),
structurally identical to the mineral realgar, which
is stable at room temperature, ii) the high temperature phase, β-As4S4,
with base-centered monoclinic crystal structure (space group C2/c) stable above
Experimental
The investigation was carried out with
mineral realgar – sample A, collected from Allchar locality (R. Macedonia) and pararealgar.
The pararealgar was prepared innovately
by milling – sample B (planetary mill Pulverisette 6,
X-ray diffraction measurements were carried out using a D8 Advance diffractometer (Bruker, Germany) equipped with a Q/Q goniometer, Cu Ka radiation (40 kV, 40 mA), secondary graphite monochromator, and scintillation detector. The diffraction data were collected over an angular range 10 < 2Q< 100° with steps 0.03° and a counting time 20 s/step. The commercial Diffracplus Eva software has been used for phase analysis according to the ICDD - PDF2 database.
The particle size distribution was measured by photon cross-correlation
spectroscopy using a Nanophox particle sizer (
Dissolution tests were conducted in simulated gastric fluid (SGF)
composed of 0.2% NaCl in 0.7% HCl
(pH = 1.3) and in a simulated intestinal fluid (SIF) composed of 0.042% NaOH, 0.4% NaH2PO4.9H2O and 0.6% NaCl
with pH 6.5 at
Results
Characterization of the materials
Realgar – sample A.
High-purity mineral, realgar,
crystallizing in monoclinic crystal structure, space group P21/n,
JCPDS 01-076-9449 (Fig. 1a).
Pararealgar – sample B and C, prepared by two alternative
pathways:
B - Milling of realgar in a
planetary mill Pulverisette 6. The XRD pattern of the
sample after milling is shown on Fig.1c, the pararealgar-b phase, crystallizing in
the base centered monoclinic system, space group C2/c, JCPDS 01-075-8666 was
confirmed (Fig.1b). Line broadening and relative intensity decrease indicate
the decrease of crystallite size and amorphization.
C - Irradiation of realgar
by sunlight during one month. Absorption of visible photons with energies in
the range 1.85–2.48 eV leads to irreversible isomerization of realgar to pararealgar, whereby the positions of one arsenic atom and
one sulfur atom in the As4S4 cluster become exchanged
[5], the process is accompanied by visible color change of the mineral from red
to yellow. The transformation, realized by structural rearrangement and
bond-breakings, leads also to considerable amorphization,
as it can be seen from the XRD pattern on Fig. 1c. The product is a
structurally non-homogeneous, multiphase system, with the main component pararealgar (monoclinic P21/c phase, JCPDS
01-083-1013) and b - phase. Non-transformed realgar
can be also detected.
The nanosuspensions were
prepared from samples A and C. The estimated average particle size x50 was
137 nm (142 nm) for the realgar (pararealgar)
nanosuspensions, respectively, and 99% of particles
were confirmed to be smaller than 200 nm. Interestingly, the main pararealgar component, present in the sample C, can not be
detected in the obtained nanosuspension, which is
composed of the majority pararealgar-b phase and of some
non-transformed realgar (Fig.2).
Dissolution in
simulated gastric and intestinal fluids
Great
rise in the solubility of arsenic was achieved by nanomilling,
the amount of dissolved arsenic after 240 minutes of leaching in SGF + SIF
increased from 2% to 12% (13.5%) for the nanomilled realgar (pararealgar),
respectively. These results are very promising with respect to the published
literature results. For comparison, only 0.6% of arsenic of the total realgar content was finally released into simulated gastric
juice in [6], whereas some authors reported that 4% of arsenic from realgar were traced in gastric and
intestinal fluids [7].
Anti-cancer effects
The nanomilled samples showed increased cytotoxicity.
The values of 50% inhibition concentration (IC50) of milled samples
to H460 cells were 0.033 (0.031) μg/mL for the nanomilled realgar (pararealgar). For
comparison, this concentration for the used anti-cancer agent, cisplatin, is 0.01 μg/mL. In general, the
results imply that the lung cancer cells are susceptible to the treatment with
these samples.
Cell cycle progression and induction of apoptotic cells
The
H460 cells were treated with various concentrations of arsenic sulfides for 24,
48 and 72 h. The cell cycle distribution was determined, monitoring the G1
(growth phase), S (DNA replication phase) and G2/M (growth phase immediately
preceding cell mitosis) phases.
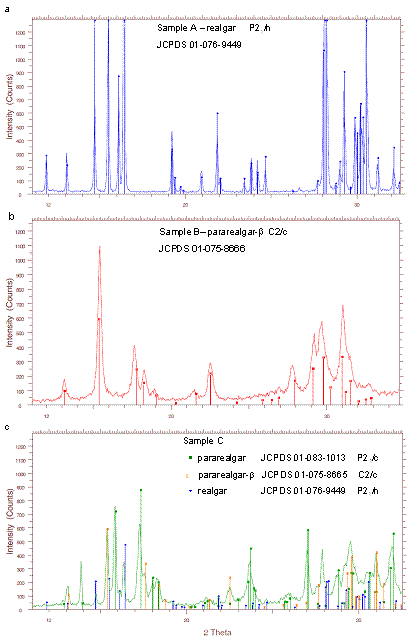
Figure 1. XRD patterns showing the phase composition of the source samples for preparation of nanosuspensions.
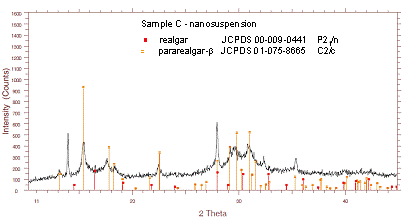
Figure 2. XRD pattern of the nanomilled sample C (light-irradiated realgar).
The treatment of H460 cells with nanomilled
A and C samples for 24 and 48 h resulted in reduction of G1 phase,
accumulation of G2/M phase, and appearance of SubG1 cells (indicating apoptotic
cells). Similarly to cisplatin, significantly
increased number of SubG1 cells was observed after 72 h, indicating the cell
cycle interference which may trigger the apoptotic pathways.
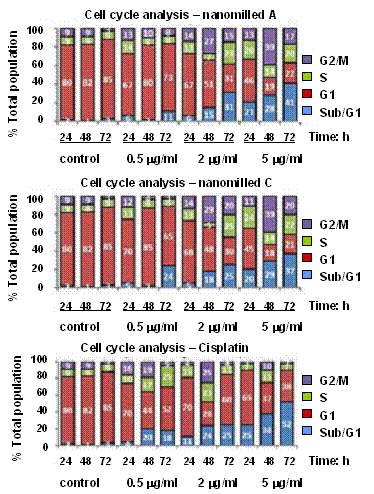
Figure 3. Cell cycle perturbation and
apoptotic cell death induced by nanosuspensions
prepared from samples A and C, and cisplatin for
comparison.
Summary
Nanosuspensions
of realgar and light irradiated realgar
(composed of pararealgar, b-phase
and realgar) with average particle size below 150 nm
were prepared in a circulation mill. The
nanosuspensions were stable for more then one month.
They have shown increased cytotoxicity and DNA damage
activity on H460 lung cancer cells, with accumulation of G2/M phase inducing
apoptotic cells.
References
1. R. Bentley, T.G. Chasteen, J. Chem. Educ. 7 (2002), 51.
2. Y. Tian, X. Wang, R. Xi, W. Pan, S. Jiang, Z. Li, Y. Zhao, G. Gao, D. Liu, Int. J. Nanomed. 9 (2014), 745.
3. P. Baláž, Z. Bujňáková, O. Kartachova, M. Fabián, B. Stalder, Mater. Lett. 104 (2013) 84.
4. L. Bindi, P. Bonazzi, Am. Miner. 92
(2007) 617.
5. P. Naumov, P. Makreski, G. Jovanovski, Inorg. Chem. 46 (2007) 10624.
6. S.Y. Kwan, S.K. Tsui, T.O. Man, Anal. Lett. 34 (2001) 1431.
7. J. Koch, S. Sylvester, V.W.M. Lai, A. Owen, K.J. Reimer, W.R. Cullen, Toxicol. Appl. Pharm. 222 (2007) 357.
Acknowledgements.
The Slovak Grant Agency VEGA (2/0064/14 and 2/0027/14), the Agency for Science and Development (project APVV-0189-10) and the European Regional Development Fund (nanoCEXmat I and II - ITMS 26220120019 and 26220120035) are gratefully acknowledged.