Structural surprises in organometallic chemistry
P. Štěpnička
Department of Inorganic Chemistry, Faculty of Science, Charles University in Prague, Hlavova 2030, 12840 Czech Republic
stepnic@natur.cuni.cz
Since its discovery in early 1950’s [1] when it was looked upon as a real structural surprise, ferrocene and its derivatives gradually spread over and influenced nearly all chemistry fields, finding applications in areas as diverse as catalysis, material design and bioinorganic chemistry [2]. Probably the most spectacular applications of ferrocene compounds were achieved in organometallic catalysis where ferrocene-based ligands, predominantly phosphines, have often played a pivotal role [2]. The numerous successful practical applications of ferrocene ligands in catalysis naturally encouraged search for new ferrocene phosphines with specific physicochemical and coordination properties and investigations into the reactions of ferrocene compounds in general.
This contribution will present some unexpected reactions of ferrocene alkynes with 6,9-(Me2S)2-arachno-
B10H12 encountered during our studies on ferrocenyl-substituted 1,2-dicarba-closo-dodecaboranes [3] and will further focus on the structurally unpredictable Cu(I) complexes resulting from the reactions of a simple phosphinoferrocene ligand 1¢-(diphenylphosphino)-1-cyanoferrocene with Cu(I) precursors (Scheme 1) [4]. In both these cases, single-crystal X-ray diffraction analysis played a vital role in elucidation of the structures of the compounds formed.
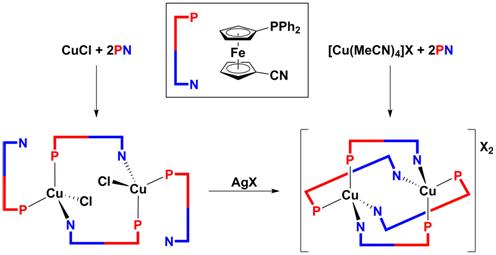
Scheme 1. Reactions of 1¢-(diphenylphosphino)-1-cyanoferrocene with various Cu(I) precursors.
1. a) P. L. Pauson, T. J. Keally, Nature, 168, (1951), 1039; b) S. A. Miller, J. A. Tebboth, J. F. Tremaine, J. Chem. Soc., (1952), 632; c) G. Wilkinson, M. Rosenblum, M. C. Whiting, R. B. Woodward, J. Am. Chem. Soc., 74, (1952), 2125; d) E. O. Fischer, W. Pfab, Z. Naturforsch., 7b, (1952), 377.
2. Ferrocenes: Ligands,
Materials and Biomolecules, edited by P. Štěpnička (Chichester: Wiley), 2008.
3. A. Korotvička,
4. K. Škoch, I. Císařová, P. Štěpnička, Inorg. Chem., 53, (2014), 568
The results presented in this contribution were obtained with financial support from the Czech Science Foundation (project nos. P207/11/0705 and 13-08890S).