Molecular
simulation of layered structures
M. Pospíšil, P. Kovář,
A. Tesař
Charles
University in Prague, Faculty of Mathematics and Physics, Ke
Karlovu 3, 121 16 Prague 2
pospisil@karlov.mff.cuni.cz
We solved the structure of various kinds of alcohol (methanol and ethanol is presented) into Sr phosphonate layers [1] with summary formula Sr(C6H5PO3)·H2O·CH3OH (C2H5OH respectively) by molecular simulations methods [2, 3]. These calculations are suitable for studying of various arrangements and positions of the methanol (ethanol) molecules in the interlayer space of the Sr host layer to obtain a detail view into mutual interactions between phenol rings of Sr layers and methanol (ethanol) in the large superstructures. Samples were prepared, described and experimentally characterized at The Joint Laboratory of SSCH at the University Pardubice and results from experimental measurements were used as a base for procedure preparation and for verification of simulation results [4, 5].
Parameterization and building of the layers was done on the base of single crystal data. Supercell of 2a x 2b x 1c was prepared and space group was changed for calculation from Pn21a (Pbcn for ethanol) to P1. Strontium layers except of phenol rings were kept rigid and methanol (ethanol) molecules were without any constraints. Water molecules were not inserted into calculation for methanol and several water molecules were calculated for structure with ethanol. Model was optimized in Compass force field [6] and charges were calculated by Charge equilibration approach [7]. Nonbonding energy was calculated by Ewald summation method [8] with accuracy 0.001 kcal/mole, cutoff 0.6 nm. Calculation was run for various initial positions and arrangements of methanol (ethanol and water) molecules with respect to Sr atoms. They were partially immersed into Sr cavities or placed just above Sr atoms in initial models. Several optimization procedures were tested with different values of pressure or temperature, values of convergence parameters, etc. The procedures and models with good agreement between calculated and experimental results are presented for two types of Sr layers.
The figure 1 shows the 2a x 2b x 1c supercell of Sr type I with the most probable arrangement of methanol and with minimum energy E = -22168 kcal/mole (Ecoul = -23845 kcal/mole; Evdw = -794 kcal/mole; Evalence = 2516 kcal/mole; Evalence-crossterm = -45 kcal/mole) and with cell parameters: 2a = 15.6153 Å; 2b = 17.1918 Å; c = 28.4910 Å; alpha = beta = gamma = 90.00 deg. Experimentally determined cell values: a = 7.9270(6) Å; b = 8.6110(6) Å; c = 28.333(2) Å; alpha = beta = gamma = 90.00 deg.
The figure 2 shows the 2a x 2b x 1c supercell of Sr type II with the most probable arrangement of ethanol. For example minimum energy related to interaction between ethanol and surroundings: E = -456 kcal/mole (Ecoul = -497 kcal/mole; Evdw = -102 kcal/mole; Evalence = 186 kcal/mole; Evalence-crossterm = -43 kcal/mole) and with cell parameters: 2a = 17.28 Å; 2b = 29.62 Å; c = 15.80 Å; alpha = beta = gamma = 90.00 deg. Experimentally determined cell values: a = 8.640(3) Å; b = 29.627(4) Å; c = 7.859(2) Å; alpha = beta = gamma = 90.00 deg.
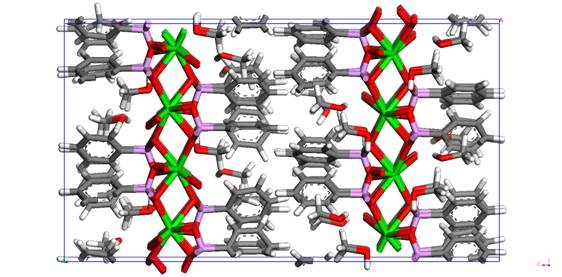
Figure 1. 2a x 2b x 1c supercell of
32 x [Sr(C6H5PO3) CH3OH] along y-axis view.
Sr is green, P is pink, O is red, C is grey and H is
white.
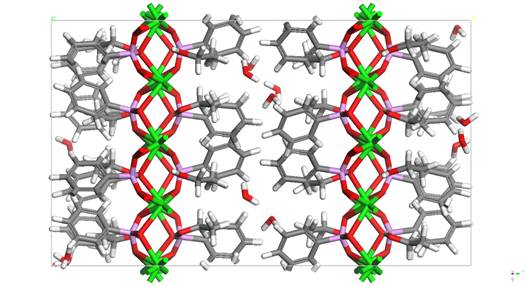
Figure 2. 2a x 2b x 1c supercell of
32 x [Sr(C6H5PO3)·(7/16)H2O·C2H5OH]
along z-axis view. Sr is green, P is pink, O is red,
C is grey and H is white.
1. A. Clearfield, K. Demadis, Metal Phosphonate Chemistry: From Synthesis to Applications, Cambridge, RSC Publishing. 2012.
2. Materials Studio Modeling Environment, Release 4.3 Documentation, Accelrys Software Inc., San Diego. 2003.
3. P. Comba, T.W. Hambley, Molecular Modeling of Inorganic Compounds, Weinheim, New York, Basel, Cambridge, Tokyo, VCH. 1995.
4. V. Zima, J. Svoboda, L. Beneš, K. Melánová, M. Trchová, Solid State Sci., 8, (2006), 1380.
5. V. Zima, J. Svoboda, L. Beneš, K. Melánová, M. Trchová, J. Dybal, J. Solid State Chem., 180, (2007), 929.
6. H. Sun, The Journal of Physical Chemistry B, 102, (1998), 7338.
7. A.K. Rappé, W.A.III Goddard, J. Phys. Chem., 95, (1991), 3358.
8. P.P. Ewald, Ann Phys (Leipzig), 64, (1921), 253.
9. N. Karasawa, W.A.III Goddard, J. Phys. Chem., 93, (1989) 7320.
This
work was supported by the Czech Science Foundation Project No. P106/14-13368S