DISORDERS: PROBLEMS OR NEW INFORMATION IN CHEMICAL CRYSTALLOGRAPHY?
J. Monco¾
Institute of Inorganic Chemistry,
Technology and Materials,
Faculty of Chemical and Food Technology,
Slovak University of Technology in Bratislave,
Radlinského 9, SK-81237 Bratislava, Slovenská Republika
jan.moncol@stuba.sk
The last decades is characterized rapid development of single-crystal crystallography. This progress can be assigned markedly expand of diffractometers with area detectors and installation of microfocused X-Ray sources. The massive expand of single-crystal structural analysis is related also with progress of software. The software progress determinate also requirement of solving crystal structures containing disorders. The quanta of disordered crystal structures correspond with growth average numbers of atoms in determined crystal structures. The most popular software for chemical crystallography is SHELX in various versions [1], which can be used in more graphical interfaces and software package, for example WINGX [2], XSEED [3], OLEX2 [4], ShelXle [5] and PLATON/SYSTEM-S [6]. The software package OLEX2 [4] can be also used for determination of crystal structure with its programs Olex2.solve and Olex2.refine. The instructions of program Olex2.refine are familiar SHELXL, but program contains also individual directions. Second usefully software package for chemical crystallography with independent utility for refinement of crystal structure is CRYSTALS [7]. However, alternative software package for determination of crystal structure of standard, modulated and magnetic samples is JANA-2006 [8]. All these programs allow determination of disorders with alternative approach of constrains, restrains and rigid-body modeling. The strategy of refinement of variable disorders have been documented relative adequately for the two most popular programs for chemical crystallography SHELXL [9,10] and CRYSTALS [10,12].
The disorder can describe as a violation of the crystal symmetry and translation. The content of the asymmetric units in disordered structures is not identical, but it is identical on average. The disorders can be decided to more groups.
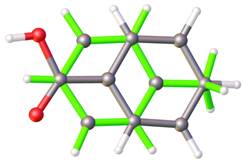
Substitutional disorders are indentified if a crystallographic position is occupied by more than one type of atom for example in minerals and ionic crystals. The problems of substitutional disorder can be solved by similar strategy in both main programs for chemical crystallography SHELXL and CRYSTALS. The relative rare examples of substitutional disorders are crystal structures containing more than one chemical different molecules in same place. On the Figure 1 is drawn example of substitutional disorders containing alternative toluene and benzoic acid molecules in same place of cell.
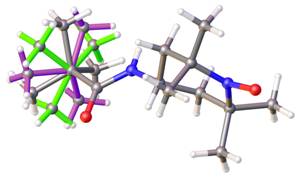
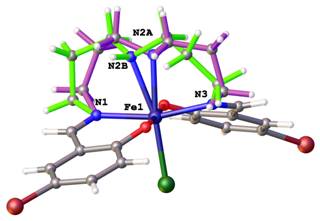
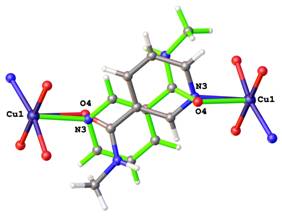
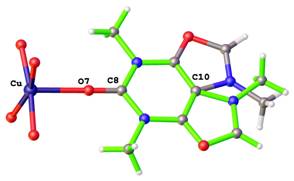
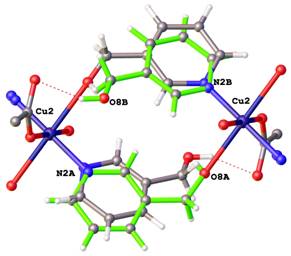
Positional disorders represent fact that an atom might be found in more than one position. They can be defined as rotational disorder, pseudorotational disorder and whole molecule disorder. Rotation disorder is presented if a group with rotational freedom might be found in two or more different rotatmers. A typical example of rotation disorder is the terc-butyl group (See Figure 2). Typical example of pseudorotational disorder is tetrahydrofurene, where saturated cycles might also be found in two conformations next to each other. On the Figure 3 is shown infrequent example of pseudorotational disorder of metalocycles in crystal structure of iron(III) Schiff-base complex. Whole molecule disorder is most often localised for co-crystallised solvents, but it can be observed in crystal structures of coordination compound, where ligands lie around special positions. On the Figure 4 is drawn example of whole molecule disorder of bridging N-methylnicotinamide ligand in copper(II) complex around special position of inversion centre. Second example of whole molecule disorder is caffeine ligand, which lies on 4-fold rotoinversion axes in copper(II) complex (See Figure 5).
The disordered groups of structures can be exist as static disorder or dynamic disorder. The rotation disorders can modeled in discrete positions using programs for chemical crystallography, but program CRYSTALS proposes also possibility of continual rotating model. The very usefully instructions for modeling disorders are rigid groups. For example ideal benzene ring can by modeled using instructions AFIX 66 in SHELXL, and \REGULARISE instructions HEXAGON or PHENYL in CRYSTALS. The regular angle instruction as well as instruction for regular tetrahedron and octahedron are allowed in program CRYSTALS. The disorders can be also modeled using rigid-body instructions in both programs. The rigid-body instructions can be defined using input atom coordinates from Cambridge Structural Database [13] or Idealized Molecular Geometry Library [14].
Sometimes a solvent molecule and/or small ion can be neither identified nor modeled. In such cases option SQUEEZE procedure [15] in PLATON [6] can be used. It is applied using three different ways. The older SHELXL-97 is refined only discrete model of molecule and SQUEEZE procedure is not refined. New version SHELXL-2014 (also versions 2012 and 2013) as well as CRYSTALS are refined co-operatively discrete model of molecule and SQUEEZE procedure. An alternative procedure, implemented in OLEX2, is based of bulk-solvent correction in large macromolecular structures [16].
The disorders can carry new chemical information. For example, the positional disorder of 3-pyridylmethanol ligand shows possibilities existences mononuclear molecular complex (forming hydrogen-bonding supramolecular chains) and coordination polymeric forms of copper(II) complex (See Figure 6). Second example new chemical information from disorder has been observed in crystal structure of iron(II) complex with 3,3'-(1,2,4-thiadiazole-3,5-diyl)dipyridine ligands. The both 3,3'-(1,2,4-thiadiazole-3,5-diyl)dipyridine ligands have been modeled using disorder of 1,2,4-thiadiazole rings, which allow two alternative possibilities of binding to iron atom. The crystal structure of iron(II) complex shows superposition of three regioselective isomers (See Figure 7).
This contribution could be shown possibilities and comparisons of two the most popular program for chemical crystallography SHELXL and CRYSTALS.
|
|
|
|
Figure 1. Substitute disorder containing toluene and benzoic acid. |
Figure 2. Rotating disorder of terc-butyl group in three positions. |
|
|
|
|
Figure 3. Pseudorotation disorder in metalocycles of iron(III) complex. |
Figure 4. Disorder of bringing N-methylnicotinamide ligand around special position. |
|
|
|
|
Figure 5. Disorder of caffeine ligand around special position. |
Figure 6. Disorder of 3-pyridylmethanol ligand forming coordination chain or hydrogen-bonding supramolecular chain. |
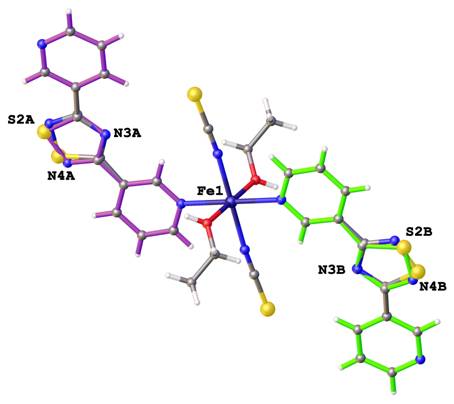
Figure 7. Disorders of 3,3'-(1,2,4-thiadiazole-3,5-diyl)dipyridine ligands forming three structural isomers ron(II) complex.
1. G. M. Sheldrick, Acta Cryst., A64, (2008), 112.
2. L. J. Farrugia, J. Appl. Cryst., 45, (2012), 849.
3. L. J. Barbour, J. Supramol. Chem., 1, (2001), 189.
4. O. V. Dolomanov, L. J. Bourhis, R. J. Gildea, J. A. K. Howard, H. Puschmann, J. Appl. Cryst., 42, (2009), 339.
5. C. B. Hübschle, G. M. Sheldrick, B. Dittrich, J. Appl. Cryst., 44, (2011), 1281.
6. A. L. Spek, Acta Cryst., D65, (2009), 148.
7. P. W. Betteridge, J. R. Carruthers, R. I. Copper, K. Prout, D. J. Watkin, 36, (2003), 1487.
8. V. Petrøíèek, D. Dušek, L. Palatinus, Z. Krist., 229, (2014) 345.
9. P. Müller, Chapter 5 - Refinement of Disorders with SHELXL, "Crystal Structure Refinement, A Crystallographer's Guide to SHELXL" by . P. Müller, Ed. Oxford University Press, 2006
10. N. S. Weng, Chinese J. Struct. Chem., 24, (2005), 1425.
11. P. V. Solntsev, Disorders Refinement Using CRYSTALS Software. 2013.
12. M. Neuburger, Disorders in Crystal Structures: New Approaches in Finding the Best Model, PhD. Thesis, University of Basel, 2011.
13. C. R. Groom, F. A. Allen, Angew. Chem., Int. Ed., 53, (2014), 662.
14. I. A. Guzei, J. Appl. Cryst., 47, (2014), 806.
15. P. v. d. Sluis, A. L. Spek, Acta Cryst., A46, (1990), 194.
16. B. Rees, L. Jenner, M. Yusupov, Acta Cryst., D61, (2006), 1299.
This paper is supported by Slovak Grant Agency (VEGA 1/0388/14).