Advances of Protein Crystallography
J. Hašek
Inst.of Biotechnology and Inst.of Macromolecular Chemistry,
Academy of Sciences of the Czech Republic, Praha, Czech Republic
hasekjh@seznam.cz
The General Assembly of the United Nations
(UN), on its 121st plenary meeting decided to proclaim 2014 as the
International Year of Crystallography. The following text contains several
quotes and sentences reproduced from the 66/284 General Assembly declaration:
“Recognizing the leading role of the International
Union of Crystallography (IUCr),
an adhering body of the International Council for Science (ICSU), in
coordinating and promoting crystallographic activities at the international,
regional and national levels around the world, ......
The humankind’s understanding of the material
nature of our world is grounded, in particular, in our knowledge of
crystallography. The impact of crystallography is present everywhere in our daily lives, in modern drug development,
nanotechnology and biotechnology, and underpins the development of all new
materials, from toothpaste to airplane components. Applications of
crystallography are critical in addressing challenges such as diseases and
environmental problems, by providing protein and small molecule structures
suited for drug design essential for medicine and public health, as well as
solutions for plant and soil contamination.
Persistent flow of Nobel prizes shows that the
crystallography is still fertile ground for new and promising fundamental
research.
Therefore, it encourages
all Member States, the United Nations system and all other actors to take
advantage of the International Year of Crystallography to promote actions at
all levels aimed at increasing awareness among the public of the importance of
crystallography and promoting widespread access to new knowledge and to
crystallography activities.
It is also worth to commemorate the sixty-fifth
anniversary of the founding of the International Union of Crystallography and
the foundation of the journal Acta Crystallographica involving presently six volumes A,B,C,D,E,F
devoted to specialized aspects of scientific research.”
Nobel prizes
awarded for crystallography or in close connection to crystallography were
already listed in a short review /1/. In this paper, we concentrate especially
on the last development of the protein crystallography, i.e. structure
determination of large biomolecular complexes in atomic resolution where the
international appreciation seems to be the highest.
Figure 1
summarizes the counts of Nobel prizes by each ten year since 1901 (the year
when the first Nobel prize was awarded). In comparison
with the chart published in [1], one can see here marks of oversampling.
Splitting the intervals to halves makes the profiles more humped but because we
know the background of each event we can follow the causes of these humps in
history.
The tremendous research on development
physical methods of crystallography lasting more than 40 years in the period 1895-1940 was appreciated by seven Nobel
prizes.
The high potential of X-ray crystallography
for many important activities of mankind was fully recognized in the period 1960-1980 when a completely new
and economically promising insight into the micro-world of molecules and
molecular machines discovered completely new view on modern technologies. New
discoveries opened completely new approach to molecular biology, revealed how
molecular machines work and showed basic principles behind function of living organisms. These discoveries were rewarded with five Nobel prizes.
Recognition of high importance of
crystallography induced large investments in the field, namely in development of technologies and
crystallography methods. Thus, we can see four Nobel prizes in 1980-2000 for development of techniques and
also four Nobel prizes for applications
in chemistry and molecular biology mostly due to the dissemination of new methodologies
and their accessibility over the whole world.
Finally, the recent development (13 years of
this century) shows that X-ray crystallography still has a high potential for
the future of mankind. Seven Nobel prizes awarded in the period
2003-2013 uniquely show high importance of the achievements. Particularly
promising seems to be the statistics in the field of protein crystallography,
where one can be fascinated by a new
Nobel prize every second year.
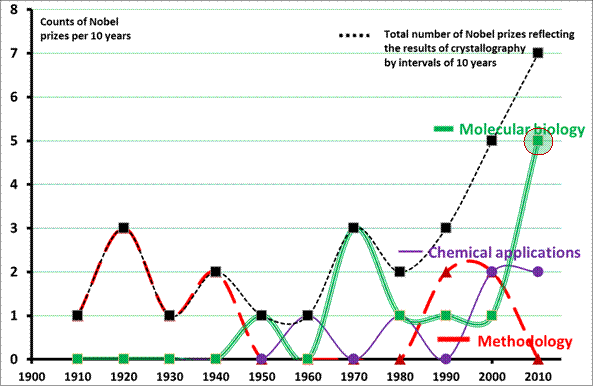
Fig.1. Numbers of Nobel
prizes closely related to applications of crystallography methods in chemistry
and biology in ten years intervals from 1900-1910 until now. The last points
count the Nobel prizes per the period 2001-2013
Why
is protein crystallography regarded so important?
Protein crystallography showed us the world
of living organisms at atomic resolution for the first time. This was necessary
to understand and rationally regulate the processes important for life. It
allowed formation of completely new fields of research and opened our eyes to
understand a huge complexity of processes in living organisms. The
illustrations in the appendix show some examples answering the questions
concerning:
·
exact
conformation and the conformational changes of the largest known molecular
complexes as e.g. ribosomes,
·
determination of intermediate states necessary for understanding the biochemical
reactions,
·
design of
completely new biomolecules showing far better performance than their
counterparts present in nature,
·
detailed
elucidation of enzymatic function and design of new catalytic centers providing
biochemical reactions allowing easy production
of rare or beforehand unavailable chemical products,
·
time resolved
analysis of bio-chemical reactions, even if it is presently restricted mostly
to processes that can be initiated or modulated by a pulse of electromagnetic
radiation,
·
analysis of intermolecular contacts, forming a base for
intercellular communication and signaling between cells in the body.
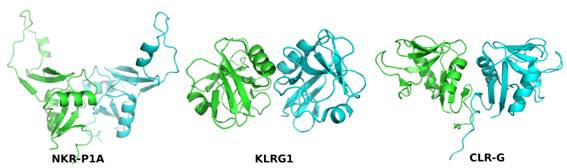
The following figure showing the supposed dimerization of receptors at the surface of natural-killer cells to accept the
information whether to start or suppress the attacks against other cells in its
neighborhood can serve as an example.
|
|
|
Figure 2. Comparison of dimerization modes of three mouse CTL proteins: NK receptor NKR-P1A (PDB code 3M9Z), NK receptor Klrg1(3FF9), and a ligand for NK receptor NKR-P1F: Clr-g (3RS1). Reproduced from [2].
Future applications of X-rays in structural biology?
The question
about future applications of X-rays in
structural biology can hardly be answered well. It seems that, after the huge
progress due to better technologies (computers, advanced detectors, synchrotron
sources of radiation) in the last twenty years, we need now to develop more
sophisticated methods for sample preparation. Here are many points, which seem
to be bottlenecks of the present research.
1.
Exact structure determination of bio-macromolecular
complexes in their native state. As a rule, the
living entities form a special state of matter. The natural environment in
living organisms where the most interesting biochemical reactions appear is not
liquid, neither solid. It is molecularly overcrowded state of matter often with
thixotropic properties, diffusion of molecules is overwhelmed by other
transport mechanisms, intermolecular interactions do not correspond to what we
can see in solutions,
etc. Here, one can see a large space for new sophisticated
procedures to prepare samples that could be inspected by X-ray crystallography.
For example new methodic for controlled preparation of realistic multi-protein
crystalline states allowing a systematic study of intermolecular contacts responsible
namely for communication between cells, for immune response, for allergy, and
for starting or modulation of processes
in living organisms.
2.
Inspection of detailed differences between similar
supra-molecular complexes and their intermediate states important for life of
different organisms. The final aim is for
example to stop some process in harmful bacteria without influencing the human
proteins or proteins in cohabiting microorganisms.
3.
Time resolved
studies. They are still in their beginnings. The problems here are not in the
diffraction technique. Standard synchrotrons allow already much better
time-resolution (~10 ns) then it is needed for biochemical reactions (µs-ms).
Shortening of the radiation pulls to femtoseconds offered by X-FEL and some new
laser facilities is harmful in this sense, because each pull destroys the
sample. The future problem is in finding
tools for immediate starting the reactions in the whole volume of our sample
and in dealing with the multiconformational states regularly observed in
the individual time sections.
The impact of
the future research is expected in many areas of human life via molecular biology, biochemistry, chemistry,
health care, drug design, agriculture, food industry, chemical industry, etc.
References
/1/ J. Hašek, International Appreciation of X-ray Crystallography. Materials Structure, 2014, 21, 4-6.
/2/ T. Skálová et al., Mouse Clr-g, a Ligand for NK Cell Activation Receptor NKR-P1F. Crystal Structure and Biophysical Properties, J. of Immunology, 2012, 189, 4881-4889.
This work was supported by the project P302/11/0855 of the Czech Science
Foundation, BIOCEV CZ.1.05/1.1.00/02.0109 from the ERDF, LG14009, and MSMT
EE-2.3.00/30.0029BIOPOL.