X-ray diffraction analysis of refractory materials used in U.S.Steel Košice
M. Černík, L. Hrabčáková, S. Štulrajter, P. Vranec
1U.S.Steel Košice, s.r.o., Vstupný areál
U.S. Steel, 044 54 Košice
mcernik@sk.uss.com, lhrabcakova@sk.uss.com, sstulrajter@sk.uss.com, pvranec@sk.uss.com,
Different types of
refractory materials are used in the refractory lining of the heat aggregates
for the steel production. Among the basic raw materials used for their
production Corundum (Al2O3), Spinel
(MgO·Al2O3), Periclase (MgO) and others are of
great matter. The refractory materials are in direct contact with the slag and
steel, where at the interface area, mainly in the case of the slag, the
refractory material is directly influenced. This influence is significantly
dependent on the type of the refractory material, operational temperature, exposition time and corrosion activity of slag and steel. Generally,
by the long-term operation the refractory material is worn, what is not the
subject of investigation, but also the structural changes take place. It can be
stated that the Corundum is stable and it does not change its crystal structure
even after a long-term exposition. Spinel-like
structures are however more interesting as the crystal structures is often
influenced by additional atoms, where the significant changes in lattice
parameters occur. Large amount of measured materials from plant including Spinel structures were divided into two groups, i.e. castables and castables
influenced by slag on its surface.
Standard lattice parameter of the Spinel MgAl2O4 with the space group Fd-3m (227) is according to ICDD card No. 82-2424 ao = 0.80887 nm and according to ICDD card No. 77-0435 ao = 0.80806 nm. Materials investigated here include real structures that are often different from the pure materials that are considered as standards. Therefore, the lattice parameter of the Spinel is in many cases different for the analyzed refractory materials containing Spinel from the plant. In many cases of these real materials the presence of two types of Spinel with different lattice parameters was identified. The Spinel structure is in these cases influenced mainly by temperature and chemical elements present in such material. It is necessary to mention that the literature describes the pressure dependence on the lattice parameter. However, our materials are not exposed to high pressures; these are operated at high temperatures and are in contact with liquid iron and liquid slag.
It has been found from many different measured and
evaluated data that the lattice parameter in the structure of material which is
in contact with liquid slag increases to higher values. The most probable
reason is the influence of the slag elements such as Na, Ca, Mg, Al, Si and Fe
on the Spinel structure. The lattice parameter takes
on higher values which decrease to the standard values by the distance change
from surface towards the basic material.
The subject of our investigation was sample
including Spinel structure with the lattice parameter
of significantly lower value than the standard. Such crystal structure is less
described in the literature. The TOPAS software was used for the determination
of phase composition content, Fig. 1. Sample 1 contained Corundum, Spinel, Calcium Aluminium Oxide
and Silicon Carbide – Moissanite. Table 1 and 2 shows
phase composition content and calculated lattice parameter of the Spinel. Table 3 shows the comparison of chemical analysis
of Sample 1 with the chemical composition calculated from the phase content. For
the simulation of the operational characteristics of the material, sample 1 was
annealed at 1500 °C 5 hour in the oxidation atmosphere. After the annealing the
surface of the sample was black (Sample 2) and few millimeters under the
surface the sample remained white (Sample 3). The content of Corundum increased
and the content of Spinel, Calcium Aluminium Oxide and Silicon Carbide – Moissanite
decreased (Table 1). The formation of Anorthite was
observed for the Sample 2. The original sample contained two types of Spinel with different lattice parameters, Table 1. First Spinel with the lattice parameter of 8.0856·10-10 m can be considered as standard Spinel
while the second Spinel with the lattice parameter of
8.0546·10-10 m cannot be considered as standard. Chemical
composition of standard Spinel with the cubic
structure can be stated by chemical formula MgAl2O4;
non-standard Spinel, non-stechiometric
MgO·nAl2O3, where n = 1.0 – 7.3 [1]. In the stechiometric crystals (n = 1.0) the Mg2+ ions
are in tetrahedral positions and Al3+ ions are mainly in octahedral
positions. When the crystal structure deviates from the stechiometric
ratio (n > 1) the excess of vacant cations is
formed mainly in octahedral positions. By the increase of Al2O3
content in the non-stechiometric Spinel
the lattice parameter decreases. The substitution of the tetrahedral Mg2+
ions by Al3+ ions can be described by the following reaction:
Mg2+ = I1/3 + Al3+,
where I1/3 are the unoccupied vacant cations.
In the limiting case the defect Spinel
correspond to γ – Al2O3. Dependence of the lattice
parameter of non-stechiometric Spinel
a = f(n) was deduced by several authors, see
[1].
Annealing in the oxidation atmosphere leads to the
reversible transformation of the Spinel to Corundum
in the extreme case. Incomplete inversion process leads to non-stechiometric Spinel MgO·nAl2O3, where the lattice parameter of the Spinel is directly dependent on n, a = f(n).
Nevertheless, there are still several questions
that are unanswered, such as:
·
How the physico-metallurgical
characteristics of the Spinel depleted by Mg change?
·
What are the physico-metallurgical characteristics of the cubic γ –
Al2O3 in contrast to Corundum for the extreme case?
·
What are the physico-metallurgical characteristics of the Spinel enhanced by elements such as Fe and others?
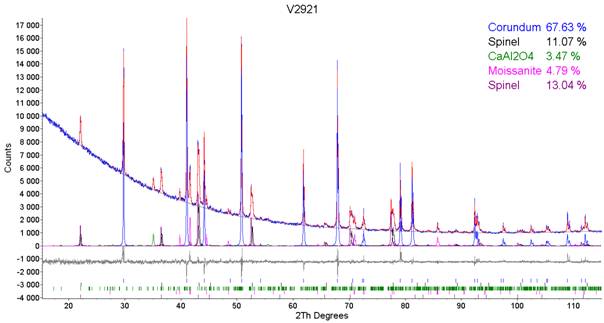
Figure 1. Sample1, detail of the refined diffraction pattern.
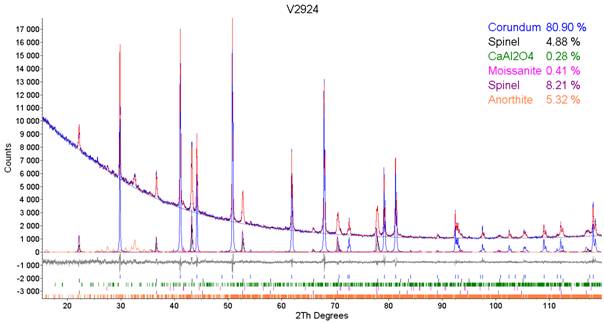
Figure 2. Sample 2, detail of the refined diffraction pattern.
Table 1. Phase compositions of the samples.
|
Identified phase composition |
Sample 1 |
Sample 2 |
Sample 3 |
||
|
Chemical
Formula |
Mineralogical
name |
Space
Group |
Content [wt%] |
Content [wt%] |
Content [wt%] |
|
Al2O3 |
Corundum |
(167) R-3c |
67.6 |
80.9 |
77.4 |
|
MgAl2O4 |
Spinel |
(227) Fd-3m |
11.1 |
4.9 |
11.1 |
|
MgAl2O4 |
Spinel |
(227) Fd-3m |
13.1 |
8.2 |
9.8 |
|
CaAl2O4 |
Calcium Aluminium Oxide |
(014) P21/n |
3.5 |
0.3 |
0.2 |
|
SiC |
Moissanite |
(186) P63mc |
4.8 |
0.4 |
1.6 |
|
CaSi2Al2O8 |
Anorthite |
(002) P-1 |
- |
5.3 |
- |
Table 2. The lattice parameters of Spinels and Corundums.
|
Chemical
Formula |
MgAl2O4 |
MgAl2O4 |
Al2O3 |
|
|
Mineralogical
name |
Spinel |
Spinel |
Corundum |
|
|
Space
Group |
(227) Fd-3m |
(227) Fd-3m |
(167) R-3c |
|
|
Lattice
parameter |
A [10.E-10 m] |
A [10.E-10 m] |
A [10.E-10 m] |
C [10.E-10 m] |
|
Sample 1 |
8.0546 |
8.0856 |
4.7592 |
12.9923 |
|
Sample 2 |
8.0504 |
8.0643 |
4.7591 |
12.9920 |
|
Sample 3 |
8.0023 |
8.0174 |
4.7589 |
12.9913 |
|
standard |
8.0898 |
|
4.75 |
12.982 |
Table 3. The
element content recalculated from phase content of samples.
|
Sample Analysis |
Al [%] |
O [%] |
Mg [%] |
Ca [%] |
C [%] |
Si [%] |
Fe [%] |
Ti [%] |
|
1 - Chem |
45.9 |
43.9 |
3.4 |
1.1 |
1.3 |
3.3 |
0.03 |
0.05 |
|
1 - X-ray |
46.2 |
44.2 |
4.2 |
0.9 |
1.5 |
3.4 |
- |
|
1 G.I. Belykh, V.T.Gritsyna ,
L.A.Lytvykov, V.B.Kolner:Transformation og spinel crystals structure MgO.nAl2O3
under high-temperature annealing, Functional Materials 216, No.3 (2009).