Fe2O3/TiO2
nanoparticles – a complex structural study
V. Valeš1,
M. Buljan2, S. Bernstorff3, S. Mangold4, V. Holý1
1Faculty of Mathematics and Physics,
Charles University, Ke Karlovu
5, 121 16 Praha, Czech Republic
2Ruder Bošković
Institute, Bijenička cesta
54, 10000 Zagreb, Croatia
3 Elettra –
Sinctrotrone Trieste S. C. p. A., Strada
Statale 14, 34149 Basovizza,
Italy
4 ANKA Synchrotron Radiation Facility,
KIT, Hermann-von-Helmholtz-Platz 1, D-76344 Eggenstein-Leopoldshafen, Germany
vales@mag.mff.cuni.cz
Titania (TiO2)-based
systems have been very intensively studied in last decades because of their
photocatalytic activity, which found broad commercial applications [1]. Functionalized titania
composites, especially Fe2O3/TiO2 systems
attracted a lot of attention recently, since they make it possible to improve
the photocatalytic performance of titania [2]. The ε-phase of Fe2O3 exhibits a very large
magnetic coercivity at room temperature so that Fe2O3/TiO2
in solutions can easily be manipulated by external magnetic field. Fe2O3/TiO2
compact thin layer composites as a photocatalyst can respond to visible light
due to the narrow band-gap of Fe2O3. The optical and
electronic parameters of Fe2O3/TiO2
nanoparticles substantially depend on the width of their size distribution.
In our previous work [3] we dealt with semiconductor
nanoparticles in amorphous silica matrix and we demonstrated that a self-ordering
mechanism of the nanoparticles occurs during the deposition of multilayers. A
spontaneous ordering of nanoparticles resulted in narrowing of the particle
size distribution. In this work we use this approach for the improvement of the
structure of Fe2O3/TiO2 nanoparticle systems,
namely we study the growth of (Fe2O3+TiO2)/SiO2
multilayers and the crystallization of the mentioned nanoparticles during
post-growth annealing. We investigated the ordering and the size distribution of the particles using grazing-incidence small-angle
x-ray scattering (GISAXS) and the inner crystalline structure by x-ray
diffraction (XRD) and x-ray absorption spectroscopy (EXAFS).
The studied (Fe2O3
+ SiO2)/(TiO2 + SiO2)/SiO2 samples
were grown by a sequential deposition, in which 10-period multilayers (with a
layers thickness 0.6, 1 or 2 nm and the SiO2 spacer thickness of 10
nm) were deposited by electron beam evaporation onto rotating Si substrates at
room temperature. The deposition rates were 10 Å/s for SiO2
and 1 Å/s for both Fe2O3 and TiO2. The
samples have been subsequently annealed for 1 h at various temperatures in
vacuum, air or forming gas (FG – Ar + 4% H2). The partial results
have already been published [4].
The powder xrd curves have
been measured by a laboratory diffractometer with a standard x-ray tube (CuKα, 1.4 kW). We used a parallel-beam setup with a
parabolic multilayer mirror in the primary beam and a parallel-plate collimator
and a flat graphite monochromator (to reduce the fluorescence signal from Fe
atoms) in the diffracted beam. The angle of incidence of the primary beam was
kept constant at 0.5 deg to suppress the substrate signal. Small angle grazing
incidence x-ray scattering has been carried out at ELETTRA synchrotron at the SAXS
beamline with the photon energy of 8 keV. The
incidence angle was a few tenths deg above the critical angle of total external
reflection. The scattered radiation was recorded by MAR image plate (2000 ×
2000 pixels). The necessary angular resolution was achieved by a large
sample-detector distance of about 1.9 m; the air scattering was suppressed by
an evacuated flight-tube. The x-ray absorption spectroscopy has been performed
at XAS beamline at ANKA synchrotron. We measured the spectra in the range 150
eV below and 650 eV above the absorption edge of both Fe and Ti in the
fluorescence mode. The measured data have been processed and analyzed by the
standard software package of the programs Athena
and Artemis.
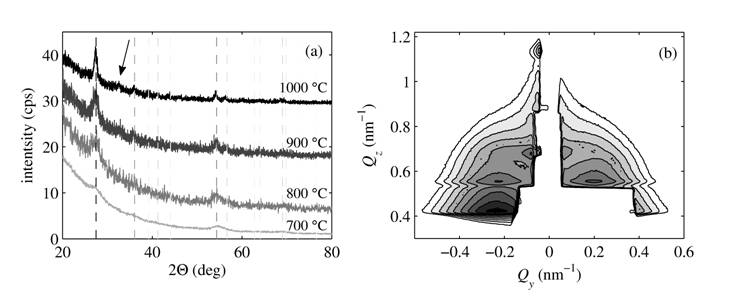
The xrd data show clearly
visible peaks corresponding to the rutile-TiO2. An example of the
evolution of the crystallinity with annealing temperature of the samples with
the layer thickness of 2 nm annealed in the air is displayed in the Fig. 1(a).
The crystallite size obtained by the Sherrer equation
applied to the 110 rutile peak exhibits a systematic growth with annealing
temperature ranging from 4 nm at 700 °C to 13 nm at 1000 °C. For the sample
annealed at the highest temperature, additional peak, which may correspond to
hematite-Fe2O3, arises.
The findings from the xrd data
have been validated by the EXAFS measurements. The absorption spectra around Ti
edge revealed the presence of rutile-TiO2 as expected. From the
measurements around Fe edge we supposed to get information on the local
structure around Fe atoms that we did not obtain form the xrd.
The data were simulated with a model assuming that the sample consists of two
components. The first component is one shell of octahedrally
coordinated oxygen atoms around an iron atom and the second one is two-shell
hematite structure. We observed a systematic increase of the two-shell
component at the expense of simple octahedrons.
The measured GISAXS maps have
been compared to the simulations carried out by our software using a model of
disordered particles in a hexagonal two-dimensional array including interface
roughness. Individual layers are expected to be laterally fully disordered due
to the fact that the particles grow during annealing after multilayer growth.
An example of nicely ordered particles (expressed by well-defined lateral
maxima in the GISAXS map) is displayed in the Figure 1(b). The results from the
GISAXS maps simulations show that with increasing annealing temperature
particle size increases as well as the degree of ordering. At the temperature
of 1000 ºC the particle arrangement as well as inter-layer structure is
broken, probably due to coalescence of particles from different layers. The
particle sizes obtained from GISAXS are in the agreement with those obtained
from xrd.
|
|
|
Figure 1. The evolution of diffraction profiles of samples annealed in the air (a) with diffraction peaks corresponding to rutile (the dashed lines, the grey scale corresponds to the relative peak intensity). The arrow denotes an additional peak corresponding to hematite-Fe2O3. In the panel (b) the GISAXS map in the logarithmic scale with the contour step of 100.15 from sample annealed in the air at 900 ºC is displayed. |
From the analysis of all the data from all
experiments we found out that titanium oxide tends to form ordered rutile
particles from the annealing temperature of 700 ºC with increasing
crystallinity and better ordering for higher temperatures. The particles are
smaller and closer together for thinner samples and vice versa. The multilayer
structure is preserved up to temperatures around 900 ºC. On the other
hand, iron atoms form only very small particles that are not recognizable by
xrd and most of the atoms is surrounded only by octehdrally coordinated oxygen atoms. The best arrangement
of (rutile) particles has been achieved for the sample annealed at 900 ºC
in the air.
1. M.
Hoffmann, S. Martin, W. Choi, D. Bahnemann, Chemical Reviews, 95,
(1995), 69.
2. H.
Cui, W. Ren, W. Wang, Journal of Sol-Gel Science and Technology, 58,
(2011), 476.
3. M.
Buljan, U. Desnica, M. Ivanda, N. Radić, P. Dubček, G. Dražić, K. Salamon, S.
Bernstorff, V. Holý, Physical Review B, 79, (2009), 035310.
4. V.
Valeš, V. Holý, M. Buljan, V. Janicki, S. Bernstorff, Thin Solid Films, 520,
(2012), 4800.