Structure
and function of bacterial nucleases
J. Stránský1,2,
J. Dohnálek2
1Faculty of Nuclear Sciences and
Physical Engineering, Czech Technical University, Břehová 7, 115 19 Praha 1
2Institute of Macromolecular
Chemistry, Academy of Sciences, v.v.i, Heyrovského náměstí 2, 162 06 Praha
6
stransky@imc.cas.cz
Keywords: nucleases, protein
crystallography, single-wavelength anomalous dispersion
Abstract
Nucleases
are a broad group of enzymes which controls hydrolysis of phosphodiester bonds
in nucleic acids. The reaction is used in wide spectrum of biological
processes, which is in correlation with number of different structures and
reaction mechanisms. Nucleases play their role in DNA replication,
transcription from DNA to RNA, nucleic acid's repairs, apoptotic processes and
controlled cell death or in degradation of nucleic acids as a nutrition source.
The reaction mechanisms are possible to characterise with respect to reaction
centre constitution, presence of metal ions, deprotonated water or typical amino-acid
residues as serine, thyrosine or histidine. One of the
bacterial nucleases was successfully crystallized and diffraction data were
collected. A phase problem solution is in progress.
Introduction
Nucleases
are a group of enzymes responsible for cleavage of DNA and RNA. The reaction is
involved in various biological processes: DNA replication, recombination,
reparation processes, nucleic acids (NA) degradation, programmed cell death,
etc. Different requirements on nucleases function leads to structural and
reaction mechanisms diversity. As nucleic acids are an essential compound of
living beings, their degradation is fatal. Therefore, production and function
of nucleases is strongly regulated in cells.
Small
bacterial nuclease (SBN) was chosen for further studies, with respect to
structure solution and reaction mechanism determination by X-ray
crystallography.
Theory and
experiment
Mechanism
of phosphodiester bond cleavage
Nuclease
catalyses disruption of one of the P – O bonds connecting units of nucleic
acids. The cleavage of phosphodiester bond is a general acid-base catalysis,
where a base activates a nucleofil by deprotonation and an acid stabilizes
final product by protonation. When activated nucleofil is close enough to
phosphate group, a highly charged intermediate is formed, where phosphorus
forms 5 covalent bonds. Finally, the scissile bond breaks.
The
nucleofile, initiated by deprotonation, is mostly a water molecule or a
hydroxyl group of serine or thyrosine, which leads to a covalent compound of
protein and nucleic acid. In this case, the complex is dissociated in the
second step. Moreover, 3'-end of nucleic acid can act as a nucleofil, resulting
in reordering of nucleic-acid chains (e.g. splicing) [5].
Bacterial
nucleases
The protein
sequence database UniProt contains 8996 sequences (291,462 unrevised; 28th
June 2013) of bacterial nucleases but there are only 645 known structures
(Protein Data Bank; 28th June 2013).
Nucleases
can be classified by several criteria. The primary criterion is the position of
the cleavage: exonucleases remove nucleotides from NA ends and endonucleases
disassemble chains to longer products. According to substrate, DNases and
RNases differs in sugar specificity, moreover, nucleases can be specific to
single-stranded (ss) or double-stranded (ds) nucleic acids. Some nucleases
prefer cleavage of given sequence of nucleotides. Number of nucleases fulfils
several options in the criteria, for example degrading nucleases show only weak
substrate specificity.
Detailed
classification can be done on the basis of reaction mechanisms. An overview of
nucleases with known structure was published by Yang, 2011 [5].
Crystallization
and diffraction measurements of SBN
Crystallization
of the small bacterial nuclease (SBN) was performed with hanging drop method.
An initial screening was held in crystallization plates, where wells were
covered by glass cover clips and sealed by silicon grease. Initial hits
occurred in the screening set Index, Hampton Research, solutions number 2 and 6
with high concentration of ammonium sulphate. Further optimization consisted of
changing of concentration of ammonium sulphate.
Crystals
were fished with nylon loops and vitrified in liquid nitrogen at 77 K. High
concentration of salt in crystallization solution serves as a cryoprotectant.
Chosen crystals were soaked in ammonium iodide.
Diffraction experiments were performed at synchrotron source BESSY II in Berlin, beamline 14.1 and 14.2 [4], using a MAR Mosaic CCD 225 or PILATUS 6M detector and mini-kappa goniometer. The wavelength of X-ray was 1.9 Å to improve anomalous scattering from sulphurs and iodines. The diffraction data were processed in XDS [2,3].
|
0.1 M Tris pH 8.5 |
0.1 M Tris pH 8.5 |
|
0.1 M sodium acetate pH 4.6 |
0.1 M
sodium acetate pH 4.6 |
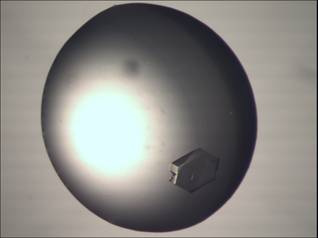
Figure 1:
Examples of crystals of small bacterial nuclease grown in solution with
ammonium sulphate.
|
|
|
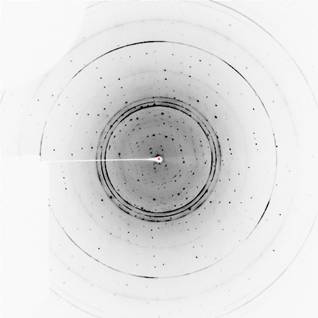
Figure 2:
Chosen diffraction frames from dataset measured on crystal with hexagonal
morphology (left panel) and needle-like morphology (right panel).
Results and
discussion
Crystals of
SBN grow in concentrations of ammonium sulphate in interval from 1.6 M to 2.4 M
(Fig. 1). The crystals usually form clusters of needles of length in order of
hundreds of micrometers and width in order of tens of micrometers. The
needle-like morphology is conserved across various conditions. In one case, a
monocrystal with hexagonal shape appeared (Fig. 1; top right).
Sample
images of diffraction data collected on these crystals are on Fig. 2. The data
often show diffraction of several lattices and in few cases powder diffraction
of water, mainly caused by ice on the surface of loop. Data with high resolution limit are usually
available. However, high resolution data were not collected yet because of
geometry limits of the experiment at wavelength 1.9 Å. The long
wavelength experiment was chosen to maximise anomalous scattering of sulphur
and iodine atoms for phase problem solution.
The data
collected on the crystal with hexagonal morphology and the needle-like crystal
soaked in ammonium iodide were processed in XDS (tab 1.)
Individual
frames measured on the crystal with the hexagonal morphology shows both strong
ice-rings and multiple crystal lattices. However, it is possible to index and
integrate reflections on the major lattice. After indexing and analysis of
systematic absences, space group P212121
was determined with cell parameters a = 47.6 Å, b = 54.1
Å, c = 32.5 Å. A signal to noise ratio (I/s(I))
is high even in high resolution shell and anomalous signal exceeds I/s(I)
value of 1 with high correlation coefficient. Low completeness in high
resolution is caused by shadows of cryo-stream nozzle and beamstop holder.
Nevertheless, the experimental phasing by SAD was not successful by now.
In the case
of the needle-like crystal, the k-axis was set to 45° to put the
crystal in more general position in the incident beam. Total 3,240° of rotation
by w-axis was collected to get high enough redundancy as expected space
group P1 was confirmed. Anomalous scattering was observed, but search
for anomalous scatterers and phasing was not successful.
Table 1: Parameters and statistics of datasets collected on crystals with
hexagonal and needle-like morphology. Numbers in brackets represent the highest
resolution shell.
|
Crystal
morphology |
Hexagonal
|
Needle-like |
|
X-ray
source |
BESSYII,
BL 14.2 |
BESSYII,
BL 14.1 |
|
Detector |
MAR Mosaic CCD 225 |
PILATUS
6M |
|
Wavelength
(Å) |
1.9 |
1.9 |
|
Detector
distance (mm) |
70 |
140 |
|
Number of
frames |
2,160 |
32,400 |
|
Exposure
time per 1 degree (s) |
0.8 |
1.5 |
|
Oscillation
angle (°) |
1 |
0.1 |
|
Space
group |
P212121 |
P1 |
|
Unit cell
parameters (Å) |
a = 47.6, b = 54.1, c
= 32.5 |
a = 22.8, b = 48.8, c
= 51.1 |
|
|
|
|
|
Resolution
limits (Å) |
47.0 –
1.85 (1.89 – 1.85) |
47.0 –
1.98 (2.03 – 1.98) |
|
Number of
observed reflections |
503,131
(7,842) |
367,755
(10,283) |
|
Number of
unique reflections |
6,893
(248) |
13,373
(625) |
|
Overall
redundancy |
73.0
(31.6) |
27.5
(16.5) |
|
Completeness
(%) |
88.1
(54.1) |
90.4
(59.1) |
|
Average
I/s(I) |
115.2
(29.5) |
17.5
(5.7) |
|
Rsym |
0.04
(0.10) |
0.20
(0.68) |
Conclusion
The small
bacterial nuclease was successfully crystallized, but the crystals with high
symmetry were not reproduced. Several datasets were collected, but the phasing
and structure solution is without any results until now. The experimental
phasing on the basis of the anomalous scattering on sulphurs is a demanding
technique, because differences between Friedel pairs are close to experimental
errors, therefore the measurement has to be optimized for this type of the
experiment. The way for improvement of the results could be an inverse beam
method or sophisticated usage of k-geometry for “true redundancy”
measurements [1].
References
1. Debreczeni, J.; Bunkoczi, G.; Ma, Q.; a spol.: In-house measurement of
the sulphur anomalous signal and its use for phasing. Acta Crystallographica
Section D – Biological Crystallography, 59, (2003): pg. 688-696.
2. Kabsch, W.: Integration, scaling, space-group assignment and
post-renement. Acta Crystallographica Section D – Biological Crystallography,
66, µ2, (2010): pg.
133-144.
3. Kabsch, W.: XDS. Acta Crystallographica Section D – Biological
Crystallography, 66, µ2, (2010): pg.
125-132.
4. Mueller, U.; Darowski, N.; Fuchs, M. R.; a spol.: Facilities for
macromolecular crystallography at the Helmholtz-Zentrum Berlin. Journal of
Synchrotron Radiation, 19, (2012): pg. 442-449.
5. Yang, W.: Nucleases : diversity of structure, function and mechanism. Quarterly
Reviews of Biophysics, 44, (2011): pg. 1-93.
Acknowledgements.
This project was supported by the
Czech Science Foundation, project P302/11/0855. The authors wish to thank Dr.
U. Müller of the Helmholtz-Zentrum Berlin, Albert-Einstein-Str. 15 for support
at the beam line BL14.1 and BL of Bessy II.