Crystal
structures of copper(II) tetracyanidoplatinates
M. Vavra, I. Potočňák
Department
of Inorganic Chemistry, Institute of Chemistry, P.J. Šafárik University,
Moyzesova 11, SK–04154 Košice, Slovakia
Introduction
The cyanide
moiety C≡N can act as a terminal or bridging ligand in cyanidometallate
complexes. In most compounds with bridging cyanide, polymeric one- (1D), two-
(2D) or three-dimensional (3D) networks are observed in the solid state, moreover
a lot of poly-nuclear zero-dimensional (0D) compounds are observed, too [1].
All compounds, which dimensionality is not 3D, are considered as
low-dimensional.
Results and
discussion
We have prepared
and structurally characterized a series of low-dimensional complexes with [CuLn][Pt(CN)4]
general composition (L are neutral polydentate N-donor ligands
and n is 1 or 2) within our study of the crystal structures and properties of
copper(II) tetracyanidoplatinates. These compounds can be divided, according to
the covalent bonds, to 0D, 1D and 2D. 0D complexes differ from each other in
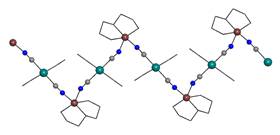
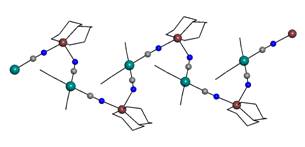
the number of metal atoms in individual complex cations. The most simple one,
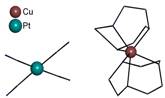
[Cu(tacn)2][Pt(CN)4]∙2H2O (1) (tacn = tridentate
1,4,7-triazacyclononane), contains mono-nuclear cation (Fig. 1a). We have also
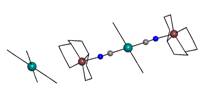
prepared two complexes with tri-nuclear cations of {[CuL]2[Pt(CN)4]}2+ composition (L are
either two bidentate 1,2-animopropane (pn)
(2) or one tetradentate
triethylenetetramine ligands (3))
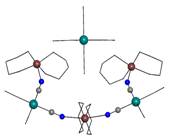
(Fig. 1b) and one complex, {[Cu(tn)2]3[Pt(CN)4]2}[Pt(CN)4]
(4) (tn =
1,3-diaminopropane), with the complicated five-nuclear cation (Fig. 1c). All
mentioned poly-nuclear complex cations contain [Pt(CN)4]2-
particles with two terminal and two bridging cyanido groups.
|
|
|
|
|
Fig. 1a: Structure of 1 with mono-nuclear
complex cation. |
Fig. 1b: Structure of 3 with |
Fig. 1c: Structure of 4 with |
The highest
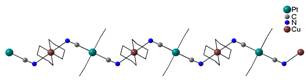
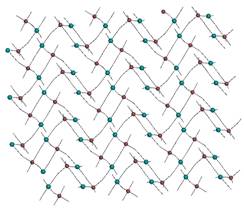
variability of the structures exhibit 1D compounds. All our prepared 1D
complexes can be described as simple chains which consist of [CuLn]2+
moieties bridged by two cyanido groups of [Pt(CN)4]2-
units. The number and a mutual position of bridging cyanido groups in the cationic
and anionic particles are specified by a pair of digits and by symbols „T”
(trans) or „C” (cis). Thus, as an example, the 2,2-TC symbol means a chain with two
bridging cyanido groups trans-coordinated
in the [CuLn]2+ species and two bridging cyanido
groups cis-coordinated in the [Pt(CN)4]2-
part [for detailed nomenclature see 2]. We have prepared compounds with all
four possible types of chains (Figs. 2a – 2d). Prepared chains are not linear
but zig-zag not only due to the cis-coordination, but also due to the
nonlinearity of the Cu–N≡C bond angles.
|
|
|
|
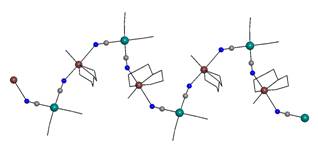
Fig. 2a: 2,2-TT chain in 5. |
Fig. 2b: 2,2-TC chain in 6. |
|
|
|
|
Fig. 2c: 2,2-CT chain in 8. |
Fig. 2d: 2,2-CC chain in 9. |
The most probable
and also most observed type of the chain is 2,2-TT type, which was
observed in six prepared compounds; as an example [Cu(en)2][Pt(CN)4]
(5) complex (en = 1,2-diaminoethane) is shown in Fig. 2a. Equatorial plane in these complexes
is occupied by four nitrogen atoms from L,
while axial positions are free for bridging cyanido groups of [Pt(CN)4]2-
particles. As blocking N-donor ligands L, two molecules of bidentate
en and its three different methyl derivatives as well as one tetradentate
ligand 1,4,8,11-tetraazacyclotetradecane were used. An interesting situation
occurs in the system with mono-methyl derivative of en (men) as two
different compounds with the same [Cu(men)2][Pt(CN)4]
composition were isolated (violet and blue crystals). The violet modification
crystallizes in triclinic P–1 space group, while the blue modification
in tetragonal P42/m
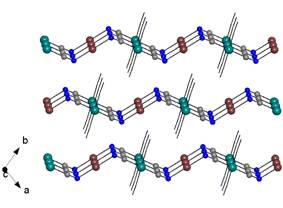
group. The most striking difference in these two structures is a
different packing of the chains. Whereas all chains in the triclinic
modification run along the [1 1 1] direction being thus mutually parallel (Fig.
3, left), the chains in the tetragonal modification are extended along both the
a and b axes. Thus two sets of parallel chains which are perpendicularly
crossed exist (Fig. 3, right).
|
|
|
|
Fig. 3: Different packing of [Cu(men)2][Pt(CN)4]
chains; left – triclinic system, right – tetragonal system. |
|
Next type
of a simple 2,2 chain is unusual 2,2-TC, observed in the [Cu(NH3)(aepn)][Pt(CN)4]∙H2O
(6) complex (aepn = 3–(2–aminoethylamine)–propylamine) (Fig. 2b).
Like in 2,2-TT,
equatorial plane of the copper atom is occupied by N-donor ligands and
bridging cyanido groups are trans-coordinated
in the axial positions. However, the bridging cyanido groups occupy cis-positions
on the platinum atom.
If N-donor
ligands occupy both the equatorial and axial positions around the copper atom,
the cis-coordination of two bridging cyanido groups is expected. Within
our research, two complexes (7 -[Cu(bpy)2][Pt(CN)4]
(bpy = 2,2`-bipyridine) and 8 - [Cu(dien)][Pt(CN)4]
(dien = bis(2-aminoethyl)amine)) of 2,2-CT type (Fig. 2c) and two
complexes (9 - [Cu(aepn)][Pt(CN)4] and 10 -
[Cu(bapa)][Pt(CN)4], where bapa is bis(3-aminopropyl)-amine) of 2,2-CC type (Fig. 2d) have been characterized. It is
important to note, that compounds with 2,2-CC chain containing [Pt(CN)4]2-
particles have not been observed in the literature up to now.
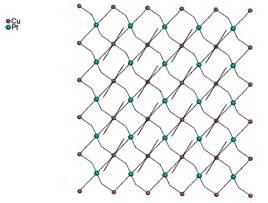
If three or
more bridging cyanido groups are bonded to the central metal atoms, 2D structures
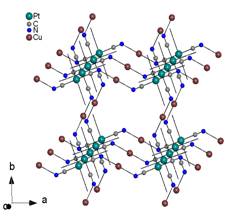
are formed and we have observed two types of different networks of the same [CuL][Pt(CN)4]
composition. If only three cyanido groups are bridging, first type of the
network is formed (Fig. 4a). Coordination number of the copper atom is five in
that case and the two remaining positions occupies bidentate ligand L,
either pn (11) or bulky tetramethyl N-derivative of en
(12). The second type is more regular because all four cyanido groups
are bridging and a square network occurs (Fig. 4b). Thus, coordination number
of the copper atom is six and last two positions occupies a molecule of
aromatic 1,10-phenanthroline, as ligand L (13).
|
|
|
|
Fig. 4a: 2D network with three bridging
cyanido groups in 12. |
Fig. 4b: 2D network with all four bridging
cyanido groups in 13. |
Conclusion
Low-dimensional
[CuLn][Pt(CN)4] complexes are according to the
covalent bonds divided into the 0D, 1D and 2D. Zero-dimensional complexes are
formed by several types of poly-nuclear ionic complexes. Each 1D complex can be
classified as 2,2 chain with two bridging cyanido groups on both copper and
platinum atoms. All four possible types of the chains were observed in our
research. 2D complexes are formed by two different types of networks with three
or four bridging cyanido groups.
References
1. F. H.
Allen, Acta Cryst., B58,
(2002), 380.
2. J. Černák, M. Orendáč, I. Potočňák, J.
Chomič, A. Orendáčová, J. Skoršepa, A. Feher, Coord. Chem. Rev. 224,
(2002), 51.
Acknowledgements
This
work was supported by the P. J. Šafárik University Internal Grant System, grant
No. VVGS 1/12-13 and by the ERDF EU (European Union
European regional development fond) grant, under the contract No. ITMS26220120047.