Crystal structures
of Fe(II) and Co(II) complexes with N-donor
bidentate ligands
L. Váhovská,
lucia.vahovska@student.upjs.sk
Keywords: iron, cobalt, spin crossover, crystal structure
Introduction
The phenomenon of spin crossover was discovered by Cambi and coworkers on series of iron(III) tris-dithiocarbamate compounds in 1930 [1]. The first Co(II) compound to show SCO, namely [CoL2]X2, where L = tridentate terimine ligand, was presented by Stoufer in 1961 [2]. This was followed by the discovery of SCO in the Fe(II) compound [FeL2(NCX)2] (X = S and Se; L = 1,10-phenanthroline, phen) by Madeja in 1963 [3]. Of all the metal ions and ligands that are known to give spin-crossover complexes, the Fe(II) and N-ligand combination leads to the greatest change in metal-ligand bond lengths between the high- and low-spin states [4]. By far the majority of known spin-transition compounds are octahedral Fe(II) compounds of general formula [FeL4(NCX)2] or [FeL2(NCX)2] (L = monodentate or bidentate N-donor ligands). The great interest is devoted to preparation of compounds with similar composition containing different neutral or anionic ligands to change the transition temperature towards room temperature. Based on these knowledge, we decided to synthesize new [ML2X2] coordination compounds, where M = Fe(II) and Co(II), L = phen, 2,2´-bipyridine (bpy) or their derivatives and X = pseudohalide anions, namely dicyanamide (dca) or tricyanomethanide (tcm). Three groups of compounds, [ML3]X2, [MLX2] and [ML2X2], have been prepared and here we report their crystal structures.
Results and discussion
Five complexes have been prepared with [ML3]X2 composition, namely [Fe(phen)3](dca)2∙4.5H2O, [Fe(5,6–dimephen)3](tcm)2 (5,6–dimephen = 5,6–dimethyl–1,10–phenanthroline) (1), [Fe(4,7–dimeophen)3](tcm)2 (4,7–dimeophen = 4,7-dimethoxy-1,10-phenanthroline), [Fe(4,7–dimeophen)3](dca)2∙1.25H2O and [Co(phen)3](tcm)2 (2). All these coordination compounds have analogous composition and consist of one [ML3]2+ complex cation and two uncoordinated anions which compensate the charge of the complex cation. In some cases, water molecules were found, too. As an example, crystal structures of 1 and 2 are shown in Fig. 1.
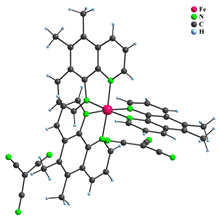
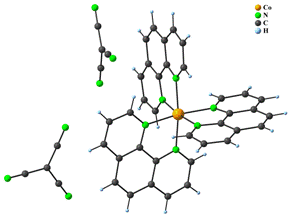
Figure 1:
Five complexes with polymeric structures, namely [Co(phen)(dca)2]n (3), [Co(bpy)(tcm)2]n (4), [Fe(phen)(dca)2]n (5) [5], [Fe(2,9-dimephen)(dca)2]n (2,9-dimephen = 2,9-dimethyl-1,10-phenanthroline) (6) [5] and [Fe(bpy)3][Fe(μ2-dca)3]2 (7) [6] have been prepared by increasing concentration of the reactants during syntheses. These compounds have similar formula, but their crystal structures differ. In 3 and 4, the Co(II) atom is hexacoordinated in the form of a distorted octahedron with CoN6 chromophore. Two nitrogen atoms originate from one neutral ligand L and remaining nitrogen atoms come from pair of bidentate anionic ligands which connect Co(II) atoms into 1D zigzag chains (Fig. 2).
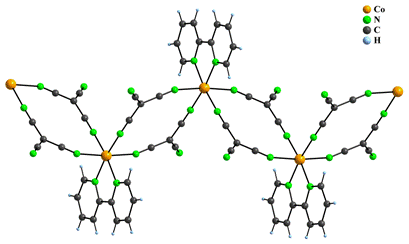
Figure 2: Polymeric chain like structure of 4
The neighbor of Fe(II) atoms in the structures of 5 and 6 is similar to Co(II) in previous structures 3 and 4 - each Fe(II) atom is coordinated by two nitrogen atoms of the ligand L and four nitrogen atoms from anionic dca ligands. However, two of these anions are coordinated in axial positions and bridge the two neighboring Fe(II) atoms forming an infinite chain. The other two dca anions are coordinated in equatorial positions and bridge the Fe(II) atoms from adjacent chains, resulting in an infinite layer (Fig. 3).
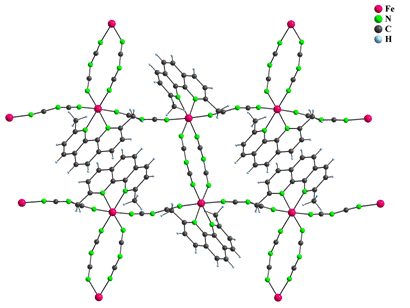
Figure 3: Part of the crystal structure of 6
Crystal structure of 7 consists of a hexagonal network in which each Fe(II) atom is surrounded by three other Fe(II) atoms, each bridged by three pairs of bidentate dca anions. This arrangement creates infinite 2D network with cavities large enough to adopt [Fe(bpy)3]2+ complex cations (Fig. 4).
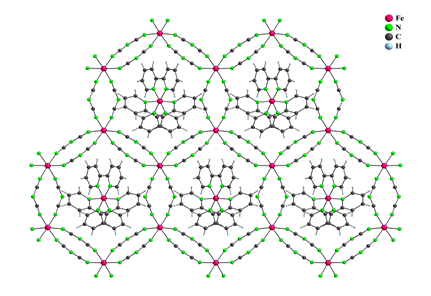
Figure 4: Part of the polymeric crystal structure of 7
The group of [ML2X2] complexes includes three compounds, namely polymorphic pair of molecular [Co(phen)2(dca)2] (8) complexes which were prepared by different syntheses, and ionic [{Co(phen)2tcm}2μ2-tcm]tcm∙H2O (9) compound. The central atoms in 8 and 9 are surrounded by six nitrogen atoms in octahedral geometry. Four of them originate from two phen ligands and remaining positions are occupied by two anionic ligands. Both dca ligands in 8 are monodentate, while only one tcm in 9 is monodentate and the second one represents a bridging ligand that interconnects two Co(II) atoms to form a binuclear cation. Its positive charge is balanced by the uncoordinated tcm anion; thus there are three different kinds of tcm bonding modes observed (Fig. 5).
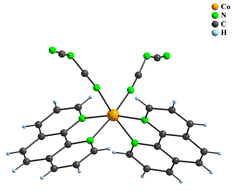
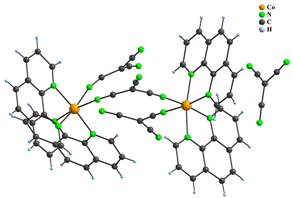
Figure 5:
References
1. L. Cambi, L. Szegö, Ber. Dtsch. Chem. Ges., 66, (1933), 656.
2. R. C. Stoufer, D. H. Busch, W. B. Hadley, J. Am. Chem. Soc., 83, (1961), 3732.
3. K. Madeja, E. König, Inorg. Nucl. Chem., 25, (1963), 377.
4. M. A. Halcrow, Polyhedron, 26, (2007), 3523.
5. D. Armentano, G. De Munno, F. Guerra, M. Julve, F. Lloret, Inorg. Chem., 45, (2006), 4628.
6. S. R. Batten, P. Jensen, B. Moubaraki, K. S. Murray, Chem. Commun., (2000), 2331.
Acknowledgements
This work was supported by the