Phase transformations of E110G Zr-alloy observed by “in-situ” XRD
J. Říha, P. Šutta
The most
important area of zirconium alloys usage is today the nuclear energetics. In
this sphere the zirconium alloys are mainly used as protective layers of
nuclear fuel rods where they create a first barrier against the reactor core
atmosphere. For this application the Zr-alloys must ensure a very low
absorption cross section for thermal neutrons, high corrosion resistance in
water steam at high pressure and temperature a good mechanical properties. In
this form these alloys are used in pressurized- and boiling-water reactors.
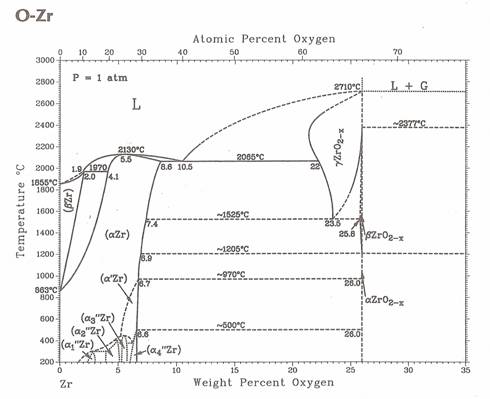
Except of those properties zirconium has a strong affinity for gaseous oxygen,
nitrogen and hydrogen with which they can form stable oxides, nitrides and
hydrides [1, 2]. Physical and mechanical properties of zirconium are influenced
especially by oxygen presence significantly. In form of solid solution oxygen and
also nitrogen stabilize the low-temperature a-Zr modification with HCP lattice and
also increase the zirconium hardness. The phase transformation temperature of pure
zirconium a ® b is
The development
of new Zr-alloys is in nowadays focused on their behaviour optimisation during
the Loss of Coolant Accident (LOCA). This type of reactor accident results in a
rapid moderator escape in time shorter than 10 seconds, followed by a rapid
heating of the Zr-alloy in steam environment at the temperature above
|
|
|
|
Figure 1. Zirconium – oxygen binary phase diagram. |
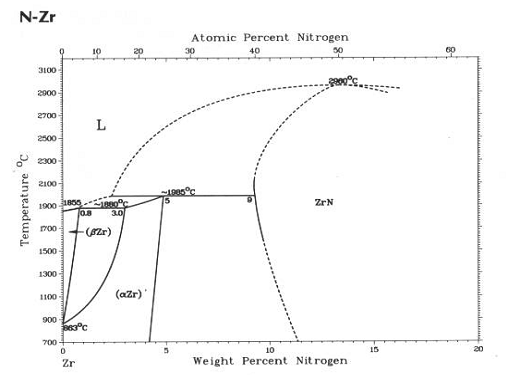
Figure 2. Zirconium – nitrogen binary phase diagram. |
The Zr-Nb
alloy E110G was used as an experimental material, Tab. 1. This material is today
most often used for nuclear fuel rods protective layers. With regard to
interstitial oxygen and nitrogen influence on phase transformations the samples
of pure Zr supplied by Goodfellow Ltd. were used for the comparison. During
previous experiments [4, 5] was observed, that the phase transformation of
zirconium to b-phase did not proceed even at
|
|
|
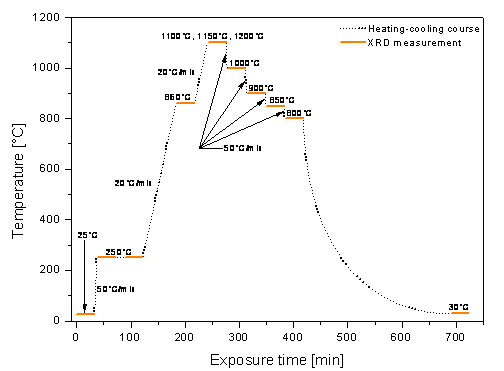
Figure 3. Heat treatment of experimental samples |
The XRD
measurements proceeded in high-temperature chamber Anton Paar HTK 1200N being a
part of automatic powder diffractometer Panalytical X’Pert Pro. This instrument
uses a copper X-ray tube (lKa = 0.15406 nm) and an
ultra-fast semiconductor detector PIXcel. The chamber was evacuated with the
aid of turbo-molecular pump Edwards EXT75DX. A dry scroll pump Edwards
XDS5 created the initial vacuum. For the lowest pressure achieving, the
deaeration step at
Table 1. Chemical composition of E110G Zr-Nb
alloy.
|
E110G Alloy |
Element |
|||||||
|
Nb [%] |
Fe [ppm/%] |
H [ppm] |
N [ppm] |
C [ppm] |
O [ppm] |
Ni [ppm] |
Hf [ppm] |
|
|
1,0 - 1,1 |
0,055 |
3 |
20 |
100 |
840 |
- |
~500 |
|
From the XRD results of both types of experimental
materials is evident that they contain a significant amount of nitrogen. This
element is in all samples in form of solid solution – diffraction patterns of
both samples in initial state show only a presence of a-Zr phase. The nitrogen causes an expressive increasing of a ® b phase transformation temperature,
Fig. 2.
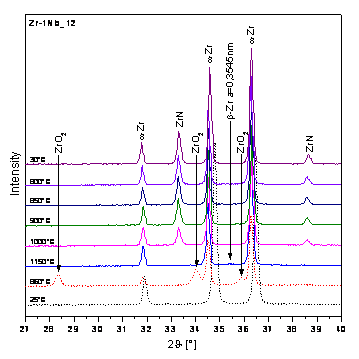
A trace
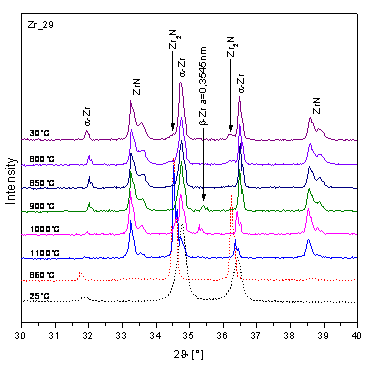
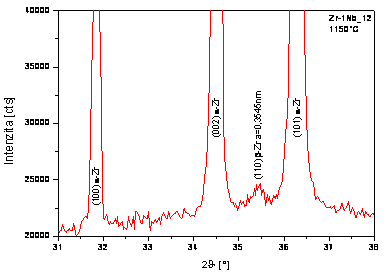
amount of high-temperature b-Zr phase can be identified in E110G
alloy after heating at
|
|
|
|
Figure 4. Partial diffraction pattern of Zr-1Nb_12 |
Figure 5. Partial diffraction patterns of Zr_29 |
|
|
|
|
Figure 5. Partial diffraction pattern of Zr_29 during the heating. |
|
References
1. M. E. Dric,: Svojstva elementov, spravočnik,
Metallurgija Moskva 1985
2. J. Koutský, J. Kočík,: Radiation damage of structural materials. Praha Academia, 1994.
3. A. R. Massih, J. Nucl. Mat., 384, (2009), pp. 330–335
4. J. Říha, O. Bláhová, P. Šutta, Chemické listy, 105, (2011), pp. 210-213
5.
J. Říha, R. Medlín, A. Vincze,
P. Šutta, Vacuum, 86,
(2012), pp. 785-788