X-ray single crystal studies of 10-arylflavins
J. Maixner1,
R. Pažout1, R. Cibulka2, J. Cibulkova1
1Central laboratories, Institute of Chemical Technology, Technická 5, 166 28 Prague 6, Czech Republic
2Department of Organic Chemistry, Institute of Chemical Technology, Technická 5, 166 28 Prague 6, Czech Republic
maixnerj@vscht.cz
Flavins (from Latin flavus, "yellow") are organic
compounds based on pteridine, formed by the tricyclic
heteronuclear organic ring - isoalloxazine. The
biochemical source is riboflavin (also known as vitamin B2). Flavins are
biologically active compounds which are responsible for redox processes in many
types of enzymes, mostly in the form of flavin mononucleotide (FMN) or flavin
adenin dinucleotide (FAD) co-factors.[1] Besides, synthetic
flavin analogues are subject of intensive research as organocatalysts of
oxidations and reductions.[2,3] The flavin group is
capable of undergoing oxidation-reduction reactions, and can
accept either one electron in a two-step process or two electrons at once.
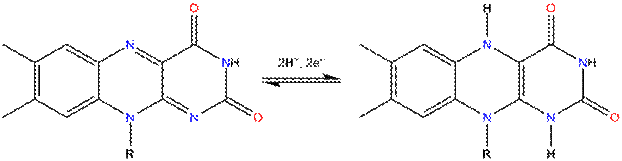
Reduction is made with the addition of hydrogen
atoms to specific nitrogen atoms on the isoalloxazine ring system:
Fl
Fl-H2
Until now, flavins have been applied for photooxidation of benzyl alcohols, benzyl amines or methylbenzenes to benzaldehydes, benzyl methyl ethers to methyl benzoates for photooxidation of dopamine, amino acids, indols, unsaturated lipids and fatty acids, glucose, fenols as well as for selective photocatalytic removal of benzylic protecting groups. The photooxidations mentioned above are usually performed in the presence of air which allows regeneration of flavin catalyst (Fl) from its dihydro form (Fl-H2). Besides hydrogen bonding, flavins are known to interact with several molecules by p-p stacking, donor p interactions and cation- or anion- p interactions. These interactions were found to be essential not only for binding flavin cofactors but also for modulating redox properties and the reactivity of flavin moieties in the biological systems. However, intermolecular interactions can negatively influence the efficiency of flavin photocatalytic system decreasing the stability of flavins or doing their redox properties more unprofitable. With the aim to minimize a readiness of flavins to aggregate, we prepared series of derivatives with ortho-substituted phenyl ring in the position 10.
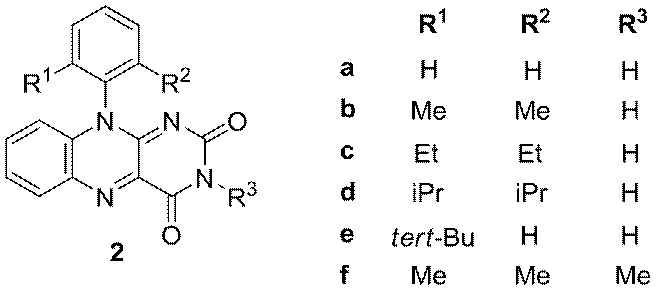
Results of X-ray single crystal works of these derivatives will be presented.
References
1. V. Massey: Biochem. Soc. Trans. 28, (2000), 283
2. F.G. Gelalcha, Chem. Rev. 107, (2007) 3338
3. V. Mojr, M.Buděšinský, R.Cibulka, T.Kraus, Org. Biomol. Chem., 9, (2011), 7257