Pentacoordinated Cu(II) complexes with tricyanomethanide, or how disappointed can be the results of structure analysis
K. Lacková,
katarina.lackova@student.upjs.sk
Keywords: copper,
tricyanomethanide, crystal structure, pentacoordination, shape of the
coordination polyhedron
Introduction
The molecular structures of five-coordinated [Cu(L)2X]Y complexes (L = 1,10-phenantroline (phen) or 2,2'-bipyridine (bpy), X and Y = 1- anions) show an extensive variability ranging from trigonal bipyramid (TBP) to square pyramidal (TEP) stereochemistries, with majority complexes displaying a structure, which is in the range of these two extremes [1]. In our previous work we have prepared and studied complexes with X = N(CN)2– and ONC(CN)2– with the aim to characterize the shape of coordination polyhedra (SCP) around the Cu atoms [2, 3]. The obtained results showed that the preferred SCP for compounds with phen is close to TBP, whereas for SCP bpy compounds is close to TEP.
Tricyanomethanide (tcm) anion C(CN)3– can coordinate similarly as N(CN)2– and ONC(CN)2– anions [4]. Therefore we decided to prepare complexes of general [Cu(L)2tcm]Y formula (L = phen or bpy and Y = ClO4–, NO3–, Br– and Cl–) and thus to continue in our previous work dealing with the SCP. In this paper we describe structures of ten ionic coordination compounds, which can be divided into three groups: a) [Cu(L)2tcm]tcm: [Cu(phen)2tcm]tcm (1) and [Cu(bpy)2tcm]tcm (2); b) [Cu(L)2tcm]ClO4: [Cu(bpy)2tcm]ClO4 (3), K[Cu(phen)2tcm]3tcm(ClO4)3 (4) and [Cu(phen)2tcm][Cu(phen)2H2O]tcm(ClO4)2·H2O (5) and c) [Cu(L)2Y]tcm: [Cu(phen)2NO3]tcm (6), [Cu(phen)2Cl]tcm·H2O (7), [Cu(bpy)2Cl]tcm (8), [Cu(phen)2Br]tcm·0,5H2O·0,5EtOH (9) and [Cu(bpy)2Cl]tcm·H2O (10).
Results and
discussion
Complexes 1 and 2 (Fig. 1) belong to the first group. Their structures are formed by [Cu(L)2tcm]+ cations and non-coordinating tcm anions. The structure of the cations consists of two phen or bpy molecules and one tcm anion coordinated to a copper(II) atom.
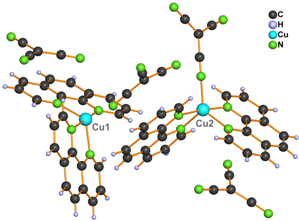
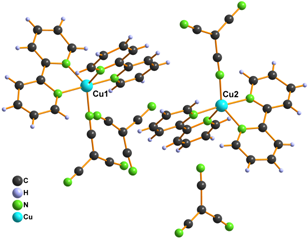
Figure 1. Structure of complexes 1 (left) and 2 (right)
The second group consists of complexes 3 - 5 (Fig. 2), which contain the same cations as the previous group, however to balance their positive charge, ClO4 in 3 or ClO4 and tcm anions in 4 and 5 are used. Moreover, structure of 4 contains K+ cation occupying threefold rotation axis and in the structure of 5 also [Cu(phen)2H2O]2+ cation and water molecule are present.
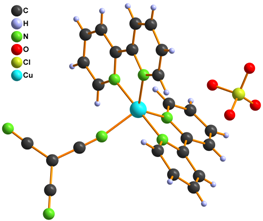
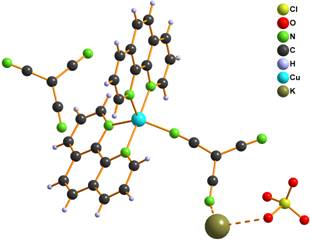
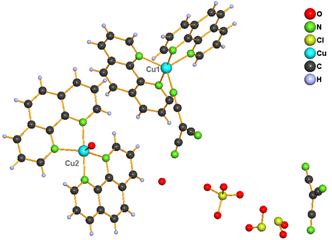
Figure 2. Structure of complexes 3 (left), 4 (right) and 5 (middle)
We also tried to prepare [Cu(L)2tcm]Y (L = phen, bpy and Y = Br–, Cl– and NO3–) complexes within our study. Infrared spectroscopy and elemental analysis were consistent with expected composition, however X-ray structure analysis disappointed us because it revealed that the prepared complexes are with exchanged anions and their formulae are [Cu(L)2Y]tcm 6 – 10 (Fig. 3).
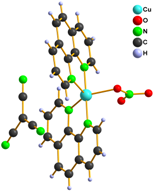
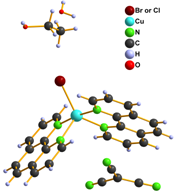
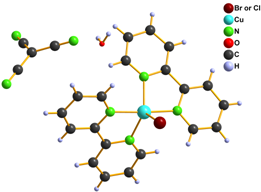
Figure 3. Structure of complexes 6 (left), 7, 9 (middle) and 8, 10 (right)
Beside the ionic forces, the structures of
the prepared complexes are stabilized by π-π interactions and,
moreover, hydrogen bonds were also observed in the structures containing water
molecules.
SCP of cations in all prepared complexes can be characterized as more or less deformed trigonal bipyramids as evidenced by the parameters τ [5] and Σ [6] (Tab. 1). Thus we can conclude that contrary to the compounds with N(CN)2– or ONC(CN)2– anions, the SCP in compounds with C(CN)3– are unaffected by the different ligands L (phen or bpy) coordinated to the Cu(II) atom.
Table 1. Summary of parameters τ and Σ(ΔTBP)
|
Complex |
τ |
Σ(ΔTBP) [°] |
|
[Cu(phen)2tcm]tcm (1) |
78,9 (Cu1) 84,7 (Cu2) |
58,2 53,7 |
|
[Cu(bpy)2tcm]tcm (2) |
77,9 (Cu1) 71,5 (Cu2) |
67,0 71,1 |
|
[Cu(bpy)2tcm]ClO4 (3) |
92,5 |
50,0 |
|
K[Cu(phen)2tcm]3tcm(ClO4)3 (4) |
76,9 |
61,7 |
|
[Cu(phen)2tcm] |
80,9 (Cu1) 90,0 (Cu2) |
59,9 44,1 |
|
[Cu(phen)2NO3]tcm (6) |
92,8 |
75,0 |
|
[Cu(phen)2Cl]tcm·H2O (7) |
72,5 |
63,7 |
|
[Cu(bpy)2Cl]tcm (8) |
69,5 |
80,9 |
|
[Cu(phen)2Br]tcm·0,5H2O·0,5EtOH (9) |
95,1 |
70,4 |
|
[Cu(bpy)2Br]tcm·H2O (10) |
86,1 |
49,8 |
References
1. F. H. Allen, Acta Cryst., B58, (2002), 380.
2.
3.
4. A.
M. Golub, H. Kõhler & V. V. Skopensko, Chemistry
of Pseudohalides.
5. A. W. Addison, T. N. Rao, J. Reedijk, J. van Rijn, G. C. Verschoor, J. Chem. Soc. Dalton Trans., (1984), 1349.
6. R. R. Holmes, J. A. Deiters, J. Am. Chem. Soc., 99, (1977), 3318.
Acknowledgements
This work was supported by the