Zirconium phase transformations measured by “in-situ” X-ray diffraction
J. Říha, P. Šutta
Zirconium
alloys are very important group of materials used in nuclear energetics. With
regard to their properties – high melting temperature, high corrosion
resistance and very low absorption cross-section for thermal neutrons the
zirconium alloys are used in pressurized- and boiling-water reactors for
protective layers of nuclear fuel rods. Except of these properties zirconium
has a strong affinity for gaseous oxygen, nitrogen and hydrogen with which it
can form stable oxides, nitrides and hydrides [1, 2]. Physical and mechanical
properties of zirconium are influenced especially by oxygen presence
significantly. In form of solid solution oxygen and also hydrogen stabilize the
low-temperature a-Zr modification with HCP lattice
and also increases the zirconium hardness. The zirconium a ® b phase transformation temperature is
The
development of new Zr-alloys is in nowadays focused on their behaviour
optimisation during the Loss of Coolant Accident (LOCA). This type of reactor
accident results in a rapid moderator escape in time shorter than 10 seconds,
followed by a rapid heating of the Zr alloy in steam environment up to
approximately
|
|
|
|
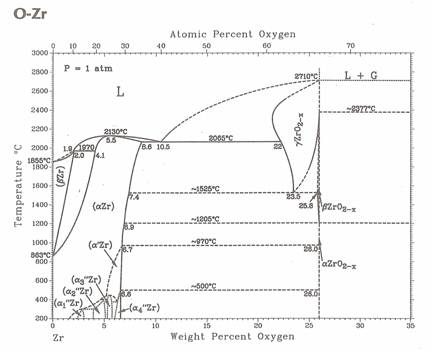
Figure 1. Zirconium – oxygen binary phase diagram. |
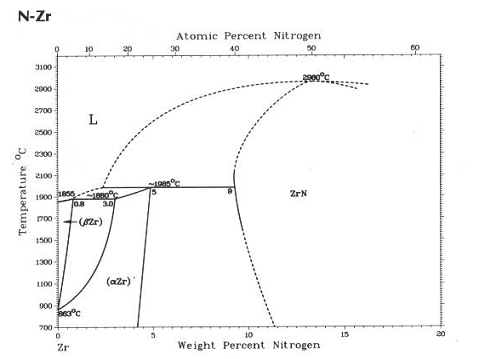
Figure 2. Zirconium – nitrogen binary phase diagram. |
Two pure
zirconium foils with dimensions 11 ´ 11 ´ 1 mm supplied by Goodfellow Ltd.
were used as experimental samples. During previous experiments [3 - 5] was observed, that the phase transformation of zirconium to b-phase did not proceed even at
Table 1. Heating courses of experimental
samples.
|
Sample |
Heating courses |
|
Zr_23 |
|
|
Zr_29 |
|
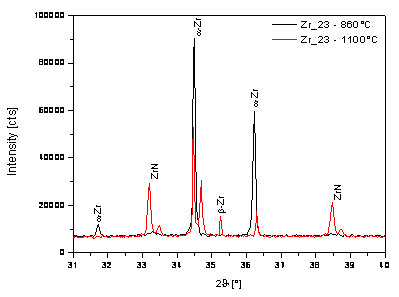
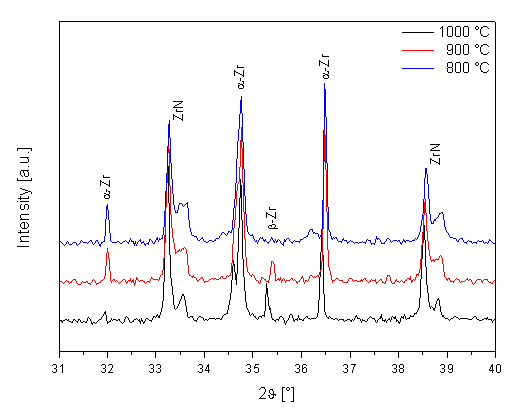
From the XRD results of both experimental
samples and also from previous experiments it is evident that zirconium foils
from Goodfellow contain a significant amount of nitrogen. This element is in
all samples in form of solid solution – diffraction patterns of both samples in
initial state show only a presence of a-Zr phase. The nitrogen causes an expressive increasing of a ® b phase transformation temperature.
The b-Zr phase could be identified at the
temperature
|
|
|
|
Figure 3. Partial diffraction pattern of Zr_23 during the heating. |
Figure 4. Partial diffraction pattern of Zr_29 during the heating. |
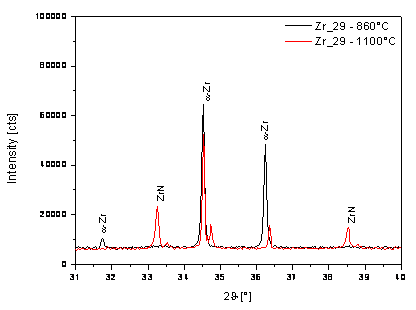
During the
cooling of Zr_29 sample, the amount of residual interstitial nitrogen amount in
the structure of zirconium is very small and the phase transformation of b-Zr to low-temperature a-phase proceeds in accord with the
binary phase diagram, Fig. 5.
|
|
|
Figure 5. Partial diffraction pattern of Zr_29 during the cooling. |
References
[1] M. E. Dric: Svojstva elementov, spravočnik, Metallurgija Moskva 1985
[2] J. Koutský, J. Kočík,: Radiation damage of structural materials. Praha Academia, 1994.
[3]
J. Říha, O. Bláhová, P. Šutta:
Fázové změny slitiny Zr-1Nb a jejich vliv na lokální mechanické vlastnosti,
Chemické listy,
[4] J. Říha, O. Bláhová, P. Šutta: Mechanical properties and structure of Zr-Nb alloy after high-temperature transformations, Chemické listy, Volume 104, Issue 15, p. 364 – 367.
[5] J. Říha, R. Medlín, A. Vincze, P. Šutta: Zirconium phase transformations observed by “in-situ” XRD analysis
[6] A. R. Massih: Transformation kinetics of zirconium alloys under non-isothermal conditions, Journal of Nuclear Materials, Volume 384, Issue 3, 28 February 2009, Pages 330-335