Structural study of titanate nanotubes
T. Brunátová1, D. Králová1,
S. Daniš1, M. Šlouf2, R. Kužel1
1Charles University, Faculty of Mathematics and Physics, Dep. of Condensed Matter Physics, Prague, Czech Republic
2Institutes of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Prague, Czech Republic
TiO2 based materials achieved broad applications. Titanate nanotubes (Ti-NT) have big surface area which is also suitable for catalytic application. Another potential application of Ti-NT is using them in lithium batteries to improve the rate of diffusion of intercalated lithium ions – small size of lithium ions and structure of Ti-NT – ionic transport in interlayer [1]. But the structure of Ti-NT is not clearly understood and different possible structures are reported. The first was the anatase structure identified by Kasuga et al. [2]. Then indications of sodium ions in the structure of Ti-NT appeared [e.g. 3, possible structure Na2Ti2O4(OH)2 or H2Ti2O4(OH)2]. Other structures reported were trititanate [4] (H3Ti2O7) or sodium trititanate (Na3Ti2O7), H2Ti2O5 H2O [5] or for example H2Ti4O9 H2O [6]. In [5], the study of dependence of Ti-NT structure on time of hydrothermal method, it was shown how the nanotubes were created – by rolling the 2D sheet.
The samples investigated were prepared at the Institute of Macromolecular Chemistry, Academy of Sciences of the Czech Republic, Prague, Czech Republic. The preparation method was a simple hydrothermal treatment of initial powder. In this work, microcrystalline rutile was used as a starting TiO2 powder. Hydrothermal method consists in boiling the initial TiO2 powder with 10M NaOH. Then obtained powder was neutralized by HCl and dried. More about preparation can be found in [7].
The samples were measured in the transmission geometry in diffractometer Rigaku Rapid II with Mo-Ka radiation, 0.3 mm collimator and 2D image plate detector. Ti-NT were measured in copper wire holder formed into a loop. Measured X-ray 2D pattern was converted into conventional powder X-ray pattern by the software 2DP.
Possible Ti-NT was identified as a beta phase of TiO2 [8]. Comparison of measured powder X-ray diffraction (PXRD) pattern and theoretical position of peaks of β-TiO2 (monoclinic, a = 12.1787 Å, b = 3.7412 Å, c = 6.5249 Å, b = 107.054° [9]) can be seen on Figure 1. Nanotube was made by rolling 2D sheet made from this structure. The variable parameters in the model were the lattice parameters and multiplicity of all the parameters except β. The multiplicity of c is given by the number of walls in a nanotube. This multiplicity could be estimated from the electron microscopy. The multiplicity of other parameters a,b have effect only on the width of diffraction peaks. The sharp peak at 2θ ~ 22° corresponds to the tube length – the tube axis is given by the parameter b and consequently its value can be determined directly. For estimation of other parameters computer simultaneous by using the Debye formula were performed.
Final simulated patterns were convoluted with the Gauss function describing the instrumental function of the diffractometer. The best agreement of calculated and experimental data achieved so far can be seen on Fig. 2.
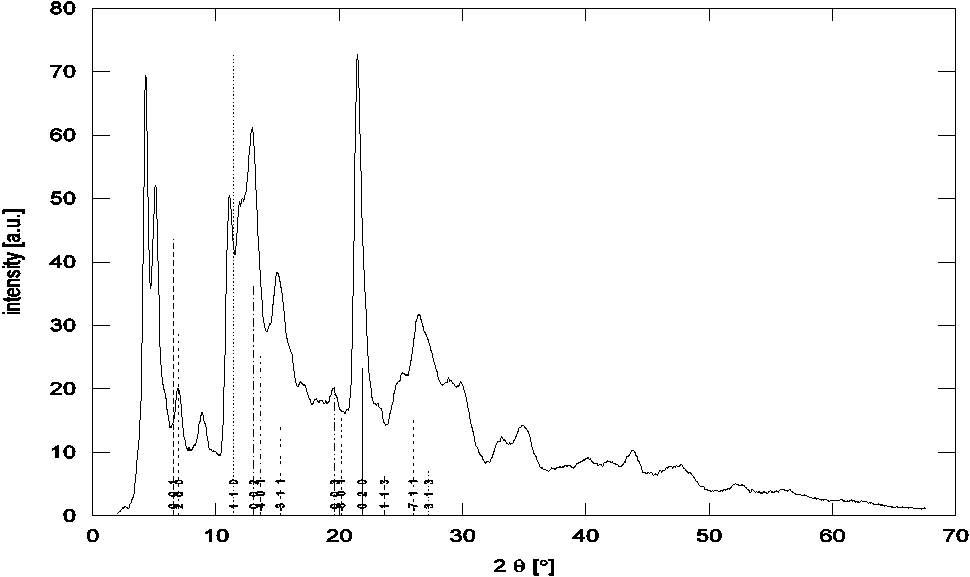
Figure 1. measured PXRD pattern and theoretical positions of peaks of
structure beta TiO2
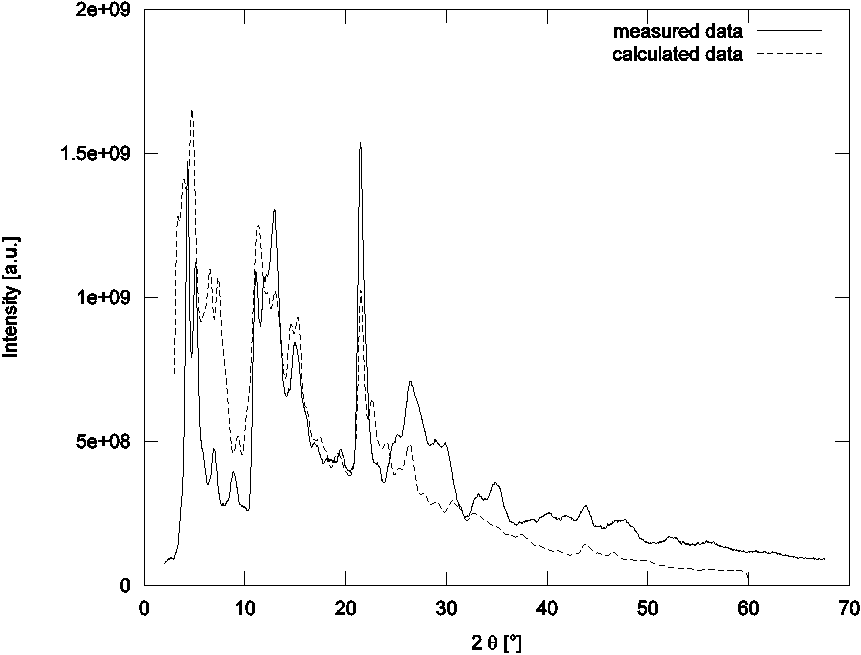
Figure 2. Comparison between measured and calculated PXRD
pattern
1. Bavykin D.V.,
Walsh F.C.:Elongated Titanate nanostructures and Their Applications, European
Journal of Inorganic Chemistry, 2009
2. Kasuga T., Hiramatsu M., Hoson A., Sekino T., Niihara K.: Formation
of Titanium Oxide Nanotube, Langmuir, 14, 1998
3. Yang J., Jin Z., Wang X., Li W., Zhang J.,
Zhang S., Guo X., Zhang Z.: Study on composition,
structure and formation process of nanotube Na2Ti2O4(OH)2,
Dalton, 2003
4. Chen Q., Zhou W., Du G., Peng L.-M.: Trititanate nanotubes made via a single alkali treatment, Advanced materials, 14, 2002
5. Chen W., Gou X., Zhang S., Jin
Z.: TEM study on the formation mechanism of sodium titante
nanotubes, Journa of Nanoparticle Research, 9, 2007
6. Nakahira
A.,Kato W., Tamai M., Isshiki T., Nishio K.: Synthesis of nanotube
from a layered H2Ti4O9 H2O in a
hydrothermal treatment using various titania sources,
Journal of materials science, 39, 2004
7. Králová D., Pavlova E.,Šlouf
M., Kužel R.:
Preparation and structure of titanate nanotubes, Materials Structure, vol. 15, 2008
8. T.Brunatova,
D. Králová, M. Šlouf, S. Daniš, R Kužel: X-ray study of
structure of TiO2 nanotubes and nanowires,
Zeitschrift fuer Kristallographie, in press
9. ICDD database PDF-4,
card number 74-1940