Complexes of Ni(II) and Pd(II) with 8-hydroxyquinoline derivatives
P. Vranec,
peter.vranec@student.upjs.sk, ivan.potocnak@upjs.sk
Introduction
Cancer is one of the major causes of death in many countries of the world. Cisplatin, as one of the leading metal-based drugs, is widely used in treatment of cancer, being especially effective against genitourinary tumours. Significant side effects and drug resistance, however, have limited its clinical applications. This perturbing news motivates us and many others to consider how to protect ourselves against cancer and as a consequence there is an increase in the design of new and more effective drugs to combat these diseases.
There are several ways how to increase the efficiency of metal organic drugs. One of them is to prepare a new drug in the form of a complex containing biologically active metal as a central atom and biologically active ligands which have adequate chelating properties and favourable toxicity profiles. This is generally true of the 8-hydroxyquinoline drug class.
One example is
5-chloro-7-iodo-quinolin-8-ol (clioquinol, CQ). CQ, a chelator of copper, zinc,
and iron, has been used for many years as an antimicrobial agent. It was first
prepared in
Interesting biological activity of 8-hydroxyquinoline derivatives (XQ) led us to an idea to prepare square-planar complexes of Ni(II), Pd(II) and Pt(II) with these ligands, which should mimic cisplatin. From the different types of syntheses we prepared 6 compounds in the form of monocrystals suitable for X-ray data collection so far. After confirming the presence of respective ligands by infrared spectroscopy and estimating composition of these complexes by elemental analysis, we studied the structures of these compounds. In this paper we describe the preparation and crystal structures of following complexes: [Ni(CQ)2] (1), NH2(CH3)2[Ni(CQ)3]·DMF·H2O (2) [Pd(CQ)2] (3), NH2(CH3)2[Pd(CQ)Cl2] (4), HCQ[Pd(CQ)Cl2]·H2O (5) and [Pd(dIQ)2] (6), where dIQ = 5,7-diiodo-quinolin-8-ol, which we expect to have an increased biological activity.
Results and
discussion
From the results of X-ray structural analysis of 1, 3 and 6 we can state that these three complexes are isostructural molecular compounds. Central atoms are coordinated by two trans-arranged molecules of XQ ligands in a square planar geometry (Fig. 1). The ligands are coordinated by nitrogen atoms of pyridine part and oxygen atoms of phenolic part after hydroxyl group deprotonation of respective ligands with M–O distances (Ni–O = 1.851(2) Å and Pd–O = 1.988(4) Å in average) slightly shorter than M–N ones (Ni–N = 1.882(2) and Pd–N = 2.004(8) Å in average) and with the distances around the Ni atom shorter than around the Pd atom.
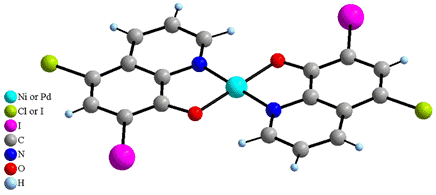
Figure
1. Structure of [Ni(CQ)2]
(1), [Pd(CQ)2] (3) and [Pd(dIQ)2] (6) complexes.
Similar differences between M–O and M–N distances have been observed in [Cu(CQ)2] and [Zn(CQ)2(H2O)]·THF·0.5H2O [2] as well as in Ni complexes with different quinolines [6, 7]. In addition, each nickel and palladium atoms in 1, 3 and 6 form long-range interactions on both sides of the molecular plane with the carbon atoms of the aromatic rings of contiguous molecules. The distances between parallel mean planes of [M(XQ)2] molecules range between 3.336 and 3.401 Å, and the distances between centroids of the aromatic rings and Ni and Pd atoms span between 3.401 and 3.477 Å (corresponding values for the [Cu(CQ)2] complex [2] are 3.316 and 3.407 Å, respectively). Such interactions give rise to stacks of molecules. Moreover the M atoms (Ni or Pd) and two adjacent centroids lie in perfect lines which make angles with the [M(XQ)2] planes of 78.8, 77.6 and 76.1° for 1, 3 and 6, respectively (Fig. 2) (76.2° in the [Cu(CQ)2] complex [2]).
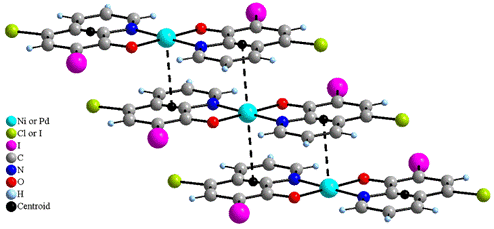
Figure 2. Stacking of the molecules in [Ni(CQ)2]
(1), [Pd(CQ)2] (3) and [Pd(dIQ)2] (6) complexes.
Collected X-ray data of 4 and 5 revealed that these two complexes are ionic and Pd(II) atoms in [Pd(CQ)Cl2]- anions are in the square-planar environment, too. Contrary to the complex 3, Pd(II) atoms are coordinated only by one CQ molecule, with Pd–O and Pd–N distances slightly longer than in 3, while the other positions are cis-coordinated by two chloride anions with average Pd–Cl distance of 2.292(18) Å. Negative charge of these complex anions is compensated by the non-coordinated dimethylammonium cation (4) or protonated molecule of CQ (5) (Fig. 3). Moreover, the structure of compound 5 contains also non-coordinated molecules of water. Both structures are stabilized by p-p interactions and hydrogen bonds involving dimethylammonium cations (4) and water molecules (5).
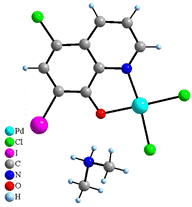
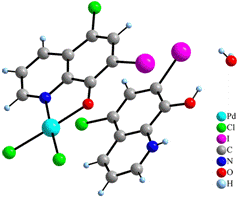
Figure 3. Structures of NH2(CH3)2[PdCl2(CQ)]
(4) (left) and HCQ[Pd(CQ)Cl2]·H2O (5)
(right).
Complex NH2(CH3)2[Ni(CQ)3]·DMF·H2O (2) is an ionic coordination compound with Ni(II) atom octahedrally coordinated by three CQ molecules in the ordinary way with N and O atoms in fac-arangement and with Ni–O and Ni–N distances adopting usual values (2.064(2) and 2.091(3) Å in average, respectively) (Fig. 4). Negative charge of [Ni(CQ)3]- anion is compensated by dimethylammonium cation. The structure of 2 also contains non-coordinated molecules of dimethylformamide and water. The structure is stabilized by weak p-p interactions and hydrogen bonds involving dimethylammonium cations and water molecules.
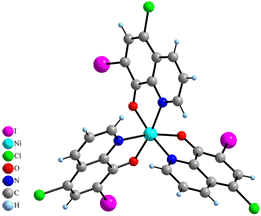
Figure 4. Structure of [Ni(CQ)3]-
complex anion (left) and NH2(CH3)2+ cation along with solvated molecules of DMF and H2O (right).
References
1. R.A. Cherny, C.S. Atwood, M.E. Xilinas, D.N. Grey, W.D. Jones, C.A. McLean, K.J. Barnham, I. Volitakis, F.W. Fraser, Y. Kim, X. Huang, L.E. Goldstein, R.D. Moir, J.T. Lim, K. Beyreuther, H. Zheng, R.E. Tanzi, C.L. Masters and A.I. Bush, Neuron, 30 (2001), 665.
2. M.D. Vaira, C. Bazzicalupi, P. Orioli, L. Messori, B. Bruni and P. Zatta, Inorg. Chem., 43 (2004), 3795.
3. D. Kaur, F. Yantiri, S. Rajagopalan, J. Kumar, J.Q. Mo, R. Boonplueang, V. Viswanath, R. Jacobs, L. Yang, M.F. Beal, D. DiMonte, I. Volitaskis, L. Ellerby, R.A. Cherny, A.I. Bush, J.K. Andersen, Neuron, 37 (2003) 899.
4. W.Q. Ding, B. Liu, J.L. Vaught, H. Yamauchi and S.E. Lind, Cancer Res., 65 (2005), 3389.
5. V. Moret, Y. Laras, T. Cresteil, G. Aubert,
D.Q. Ping, Ch. Di, M. Barthe´le´my-Requin, Ch. Be´clin, V. Peyrot, D. Allegro,
A. Rolland, F.D. Angelis, E. Gatti, P. Pierre, L. Pasquini, E. Petrucci, U.
Testa and J.L. Kraus, European Journal of Medicinal Chemistry, 44
(2009), 558.
6. S. García-Granda, P.T. Beurskens and H.J.J.
Behm, Acta Cryst., C43 (1987), 39.
7. S. García-Granda, C. Jansen,
P.T. Beurskens, H.J.J. Behm and Gómez-Beltrán, Acta Cryst., C44 (1988),
176.
Acknowledgements.
This work
was supported by the grant of the