Changes in
Phase Composition of NaAlH4+FeCl2 Complex Hydride Exposed
to Air
P. Roupcovį1, 2, O. Schneeweiss1.
1Institute of Physics of Materials,
Academy of Sciences of Czech Republic v.v.i., Zizkova 22, 616 62
2 Institute of Material Science and
Engineering, Faculty of Mechanical Engineering,
roupcova@ipm.cz
Keywords: Mössbauer spectroscopy, X-ray
diffraction, hydrogen absorbing materials.
Abstract
Phase composition in AlNaH4
doped by FeCl2 was studied by Mössbauer spectroscopy and X-ray
diffraction and stabilities in Ar and air are compared. The results show that
the hydride disappeared during the exposition to air. The major sodium alanate
hydride is trapping gaseous impurities and transformed to the Na2CO3
and Al(OH)3.
Introduction
Alanate
hydride (AlNaH4) is an important industrial material with
outstanding physical and chemical properties. AlNaH4 can be used as
a re-hydrogenated as well as an environmental friendly material. Owing to their
high storage capacity and relatively low temperature of recharging, AlNaH4-based
complex hydride have been widely applied in hydrogen tank, fuel cells, MH battery,
etc. [1-3] Additives of transition metal moderate the working temperature;
kinetics of dehydrogenation and do not changed the hydrogen storage capacity
[1-3]. The capacity of AlNaH4 catalyst by 5 mol. % FeCl2 is 4.65 wt.
% (1st thermolysis) and 2.13 wt. % (2nd thermolysis) [1, 2]. Thermal
decomposition of this material is work at around
In this
paper we compare properties of dry milled AlNaH4 and 2 mol % FeCl2•4H2O
powders in argon atmosphere and in air.
Experimental
details
The complex
hydride sample was mixed by dry milling of commercial pure AlNaH4 (Alfa
Aesar) and 2 mol % FeCl2•4H2O powders in argon
atmosphere and in air. After the milling in Ar the powder was sealed in a
plastic bag capsule filled by Ar.
The X-ray
diffraction (XRD) and Mössbauer spectroscopy (MS) were applied for
characterization of the structure of the as-prepared (before milling) powder,
and after 0.5; 1 and 2.5 hours of milling in a protective (Ar) or in ambient
atmospheres. XRD was carried out using X’Pert diffractometer and CoKα
radiation with qualitative analysis by HighScore® software and the JCPDS PDF-4
database. For a quantitative analysis HighScore plus® with Rietveld structural
models based on the ICSD database was applied. 57Fe Mössbauer
spectra were measured using 57Co/Rh source in standard transmission
geometry with detection of 14.4 keV γ-rays. The velocity scale was
calibrated with a standard α-iron foil at room temperature. Isomer shifts
δ are given relative to α-Fe at room temperature. The computer
processing of the spectra was done using CONFIT package [5] which yielded
intensities I of the components (atomic fraction of Fe atoms), their hyperfine
inductions Bhf, isomer shifts δ, quadrupole splittings ΔEQ,
and quadrupole shifts εQ.
Results
The significant
differences were observed by means the MS in the sample milled in argon
atmosphere and in air. Two main components can be recognized in the spectrum of
the sample milled in Ar. The first component – doublet with δ=1.15 mm/s
and ΔEQ=2.32 mm/s – can be ascribed to FeCl2 in
agreement with [6] and the second component – doublet with δ=0.31 mm/s and
ΔEQ=0.99 mm/s – represents of FeOCl [7]. Different two
components can be analyzed in the spectrum of the sample milled in air. The
doublets have following parameters: δ=0.17 mm/s and ΔEQ=0.55
mm/s and δ=0.5 mm/s and ΔEQ=0.63 mm/s. These values are
close to Fe(III) and Fe(III-II) in iron oxides and they probably represent
paramagnetic iron bearing oxides spread in the matrix. The disappearing of the
doublets of iron chloride and iron oxychloride can be explained by their
further oxidation during milling and/or by a chemical reaction with sodium
alanate.
XRD
measurement taken in Ar does not indicate any changes in phase composition
although a gas desorption was observed in the powder stored in Ar after milling
by expansion of the plastic bag capsule. The diffractions of NaAlH4 were only
detected there. The XRD taken on the sample after milling in air (Fig. 1)
shows formation of sodium carbonate Na2CO3 and
amorphisation of remaining phases in dependence on time. Na2CO3
replaced sodium alanate hydride taking CO2 from the surrounding air.
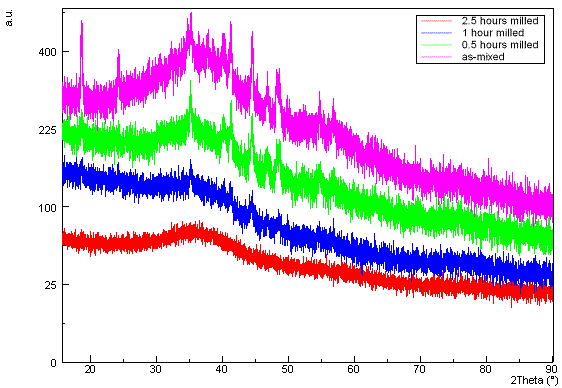
Figure 1. Comparison of XRD diffractograms of
the as-mixed sample and the samples after milling in air for 0.5, 1, and 2.5
hours.
Conclusion
The exposition of AlNaH4 doped by iron chloride to air cased changes of
chemical, phase and structure composition in all steps of sample preparation.
This material chemically reacts with gaseous CO2 in ambient
atmosphere and the Na2CO3 phase is formed. The results of
MS show that the original FeCl2 phase is oxidized and/or chemically
reacts to new iron oxide phases.
References
1. T. Kiyobayashi, S. S. Srinivasan, D. Sun
and C. M. Jensen, J. Phys. Chem A 107, (2003), 7671.
2. B. Bogdanovic, R. A. Brand, A. Marjanovic
et al., J. Alloys Comp. 302, (2000) 36.
3. Tai Sun, Bo Zhou, Hui Wang, Min Zhu, J. Alloys Comp. 467, (2009) 413.
4. B. Bogdanovic, M. Felderhoff, A. Pommerin
at al., J. Alloys Comp. 471, (2009) 383.
5. T. ˇįk, in Mössbauer Spectroscopy in Materials Science, edited by M.
Miglierini and D. Petridis, (
6. D. J. Simkin, Phys. Rev. 177, (1969),
1008.
7. Yao-Dong Dai, Zhi Yu, Hong-Bo Huang, Yun
He, Ting Shao, Yuan-Fu Hsia, Mater. Chem.
Phys. 79, (2003) 94.
Acknowledgements.
This work was supported by the Czech
Ministry of Education, Youth and Sports (1M6198959201),