Neutron
diffraction experiments on MEREDIT instrument
Přemysl
Beran
Nuclear
Physics Institute of ASCR and Research Center Řež, Ltd., 25068 Řež, Czech
Republic
pberan@ujf.cas.cz
Introduction
Neutron
powder diffractometer called MEREDIT is available for general and dedicated
research use in the Nuclear Physics Institute in Řež from the beginning of the
year 2009. Since that time we have made some very interesting measurement using
this instrument together with application of all available sample environments
which the diffractometer is equipped with. Unfortunately most of the demand for
the beam time came from the foreign institutions. So the purpose of this
presentation is to bring to Czech research community the information about the
the neutron powder diffraction instrument what is accessible “at home” and
demonstrate on several examples what the neutrons diffraction can do and
what kind of problems can help with in point of view of the structure.
MEREDIT
instrument
MEREDIT
instrument is placed on the horizontal channel number 6 in the experimental
hall of the light water reactor LVR15 in Řež. The layout of the instrument is
shown on the Fig. 1. Three different wavelengths of the neutrons available for
the diffraction experiments can be selected by two automatically exchangeable
monochromators. The details about the secondary neutron beams is written in the
Tab. 1. The multi-detector bank consists of 35 individual 3He
detectors in front of each is 10' Soller collimator. The diffraction pattern
can be collected from 2° up to 148° in 2q with different
step size.
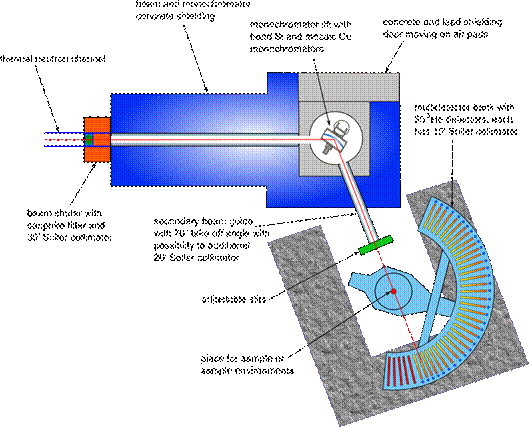
Figure 1. Layout of the MEREDIT instrument.
Table 1. Monochromators and secondary beam
details
|
Monochromator |
Reflexion |
Wavelength (Å) |
Minimum Dd/d (x10-3) |
Neutron flux (n.cm-2.s-1) |
Beam size |
|
3 bent Si single crystals |
(422) |
1.271 |
3.9 (at 56° 2q) |
~8.8x105 |
2x4 cm2 |
|
(311) |
1.877 |
4.4 (at 59° 2q) |
~8.6x105 |
2x4 cm2 |
|
|
3 mosaic Cu crystals |
(220) |
1.460 |
4.9 (at 71° 2q) |
~3.6x106 |
4x4 cm2 |
For
dedicated measurement use the instrument is equipped with different sample
environments. Vacuum furnace covers the temperature range from room temperature
up to 1000 °C and close cycle cryostat goes from room temperature down to 10 K.
Carousel for placing up to 6 powder samples and Euler goniometer are also available.
A special deformation rig permits in-situ measurements under uniaxial
stress/pressure or fatigue cycles. More information about the instrument,
sample environments, resolution function etc. can be found on the web page of
the instrument [1].
Advantages
and disadvantages of using the neutron diffraction
The neutron
diffraction if very useful probe technique because of these main advantages:
·
deep
sample penetration
·
“see”
the light elements
·
distinguishing
of elements lying beside in periodic table
·
direct
interaction with magnetic structure
There also
exist disadvantages. The main disadvantages are that for the neutron
diffraction experiment you need a large quantity of the sample and the neutron
flux is not so high – long counting time.
Let
demonstrate on the real examples measured on MEREDIT instrument the above
mentioned advantages and try to compare the results with X-ray diffraction
experiments.
Deep sample
penetration
This
advantage allows you to use vast kind of closed sample environments (furnace, cryostat,
magnet, etc.). In other hand you will collect the information from the whole
volume of your sample – useful when studying internal changes in the sample
(residual material stress/strain) or want to use non-destructive internal probe
for ex. cultural heritage investigation [2].
“See” light
elements
In some
cases important properties of material
is bind with the light elements such as O, N, C or H(D). Such kind of elements
is hardly possible to “see” by X-ray diffraction but neutrons provide very powerful
tool to describe them. In the Fig. 2 are electron and nuclear density
calculated from the X-ray and neutron powder diffraction data of lanthanum
silicate, respectively. It is clearly visible that the “visibility” of the
oxygen atoms which is crucial for this oxygen-ion conductive material is
limited in the case of the X-ray diffraction. The hydrogen storage materials
are also very important kind of materials what can be studied by the neutron
diffraction. Especially in the point of view of content of the hydrogen that is
enter to the structure. Example of such investigation is made on the
intermetallic compound ScAl0.8Mg0.2 [3].
Distinguishing
of elements lying beside in periodic table
Scattering
amplitude for X-ray increases with the atomic number but in the case of
neutrons it is not dependent on position in the periodic table. In some case as
for ex. Mn or Ti the scattering amplitude is even negative. It means that we
are able distinguish two atoms beside in periodic table (Fe/Mn) and say if
these atoms are in the structure ordered or not. The example is given in the
ferromagnetic material FeMn(Si0.5P0.5) studied by X-ray
and neutron diffraction.
Direct
interaction with magnetic structure
Due to the
fact that neutron posses the spin what can interact with the spins in the
sample we can get by using neutron diffraction the information about the
magnetic structure. Even the neutron diffraction can solve the complex magnetic
structures the example of how the spin arrangement is “visible” by neutrons is
made on simple structure of FeS. FeS shows the antiferromagnetic structure with
propagation vector k=(0, 0, 0).
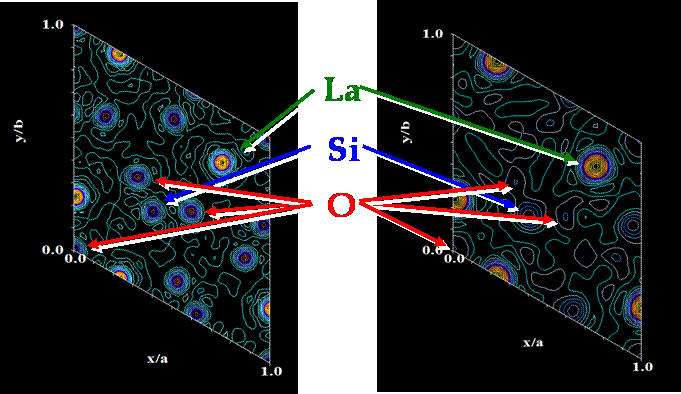
Figure 2. Nuclear (left) and electron (right)
density map of the plane (001) at z = ¼ of the lanthanum silicate.
Conclusion
Contribution
to solve some kind of problems by using the neutron powder diffraction can be
very significant. So don't think of neutron powder diffraction as a competitive
method to the X-ray or electron diffraction but as a complementary tool which
can push you further in your investigation. Moreover notice that we have such
tool now in the Czech republic and it is open for collaboration.
References
1. http://neutron.ujf.cas.cz/meredit
2. lecture at Struktura 2010 of Lubomír
Smrčok, Institute of Inorganic Chemistry of SAS, Bratislava, Rtg a neutrónová
fázová analýza produktov korózie rímskej jazdeckej helmy, June 14, 2010, 5 p.m.
3. M.
Sahlberg, P. Beran, T. Kollin Nielsen, Y. Cerenius, K. Kadeas, M.P.J.
Punkkinen, L. Vitos, O. Eriksson, T.R. Jensen, Y. Andersson,, J. Sol. State Chem. 182 (2009) 3113–3117
Acknowledgements.
Author
would like to thank for financial support from Research Centre Řež
(MSM2672244501).