An useful disorder
Jana Klimentová, Pavel Vojtíšek*, Jan Kotek
Department of Inorganic Chemistry, Faculty of Science, Charles University, Hlavova 2030, Prague 2, 128 43, Czech Republic, * pavojt@natur.cuni.cz
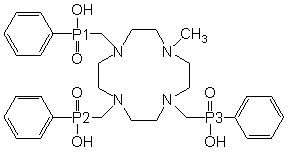
Coordination chemistry of lanthanide ions with N,O-macrocyclic ligands is widely investigated because of the importance of their medicinal and biochemical use, such as Gd3+ complexes as contrast agents (CA) in magnetic resonance imaging (MRI) [1,2], 90Y complexes in radioimmunotherapy [2] and the Eu3+ and Tb3+ compounds as the luminescence probes [2,3]. Previously, we published [4] an interesting set of structures of complexes of 1,4,7,10-tetraazacyclododecane-10-methyl-1,4,7-tris(methylene-phenylphosphinic) acid (H3L1, see Figure 1). This acid forms neutral dimeric complexes [Ln(L1)(H2O)n]2·xH2O·yMeOH with Ln3+ ions, where n = 0 or 1, x = 5–7 and y = 0–2, for different Ln3+ [4]. All compounds are isostructural and crystallise in space group P21/c (no. 14) with similar values of lattice parameters and similar position of the lanthanide ion in the unit cell.
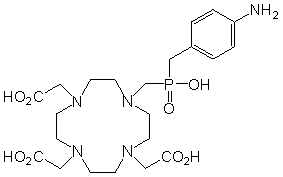
Figure 1: Structures of the ligand discussed.
|
|
|
|
H3do3pPh = H3L1 |
H4do3apABn = H4L2 |
However, the compound [Pr(L1)(H2O)1.33]2·27.5H2O does not belong to the previously published isostructural set. The structure investigation of [Pr(L1)(H2O)1.33]2·27.5H2O has resulted in an interesting conclusion [5]. The successful modelling of disorders in the case gave us a picture of “frozen solution” with samples of isomeric species present. Three chemically and four crystallographically different complexes were identified and structurally characterised:
[Pr(L1-R,S)]2 with coordination number (CN) 8, [Pr(L1-R,R)(H2O)]2 with CN 9 and two species [Pr(L1-R,S)(H2O)]2 with CN 9, but with a little different geometrical parameters (the symbols L1-R,R or L1-R,S mean the same or different chirality on ligand phosphorus atoms P1 and P2 in the complexes (P3 belongs to a bridging phosphinic group). Therefore, the title compound should be written as 0.67[Pr(L1-R,R)(H2O)]2·0.33[Pr(L1-R,S)(H2O)]2·0.67[Pr(L1-R,S)]2·0.33[Pr(L1-R,S)(H2O)]2·27.5H2O.
Compound [Y(HL2)(H2O)][Y(HL2)]·6H2O·iPrOH, where H4L2 is 1,4,7,10-tetraazacyclododecane-1,4,7-triacetic-10-methyl(4-aminobenzylphosphinic) acid (Figure 1), was prepared in the solid state and studied using X-ray crystallography [6].
In contrast to all single-crystal structures of complexes of other H4dota-like ligands published before, the structure has been “disordered” also in the macrocyclic part. The successful modelling of disorders in this case showed three distinct units found in one cell: two species adopting a twisted-square antiprismatic configuration with (TSA – CN 9) and without (TSA’ – CN 8) coordinated water molecule, [Y(HL2)(H2O)], [Y(HL2)], and one isomer with a square antiprismatic configuration (SA – CN 9), [Y(HL2)(H2O)]. In addition, this is the first complex with the H4dota-like ligand for which the structures of three possible species were determined in the solid state [6]. It can be interpreted again as a picture of “frozen solution” with samples of isomeric (conformeric) species present.
In our opinion, both examples mentioned above illustrate that disorder can represent not only a nuisance in structure solving and refinement; it may bring useful chemical information as well.
[1] The Chemistry of Contrast Agents in Medical Magnetic Resonance Imaging, eds. A. E. Merbach, É. Tóth, Wiley, Chichester, U. K. (2001); Topics in Current Chemistry, Springer, Frankfurt am Main, Germany 221 (2002).
[2] D. Parker, in Comprehensive Supramolecular Chemistry, ed. J.-M. Lehn, Pergamon, Oxford, 10 (1996) 487.
[3] S. Faulkner, J.L. Matthews, in Comprehensive Coordination Chemistry II, eds. J.A. McCleverty, T. J. Meyer, Elsevier, Amsterdam 9 (2004) 913 and refs therein.
[4] J. Rohovec, P. Vojtíšek, I. Lukeš, P. Hermann, J. Ludvík, J. Chem. Soc., Dalton Trans. (2000) 141–148.
[5] J. Klimentová, P. Vojtíšek, J. Mol. Struct. 826 (2007) 82–88.
[6] J. Kotek, J. Rudovský, P. Hermann, I. Lukeš, Inorg. Chem. 46 (2006) 3097–3102.