Polymorphism, isomorphism, distortion and
supramolecular isomerism of complexes [Cu(RCOO)2(dena)2(H2O)2]
(dena = N,N-diethylnicotinamide)
J. Moncol1,
M. Koman1, D. Valigura1
1Department
of Inorganic Chemistry, Faculty of Chemical and Food Technology, Radlinského 9,
SK-812 37
2Second affiliation, address (style Mat_address)
jan.moncol@stuba.sk
Keywords: crystal structure, polymorphism, isomorphism, supramolecular isomerism, distortion isomerism
N,N-Diethylnicotinamide (dena) [1] is an important respiratory stimulant. It is metabolized rapidly to nicotinamide, one of metabolite of vitamin B3 – niacin. The coordination of the dena ligand occurs via the pyridine ring nitrogen atom and other possible donor atoms for coordination bonds are less preferred. This preferential bonding can be seen on the basis of the structural data found in the CSD database [2]. Some copper(II) carboxylato complexes with N,N-diethylnicotinamide were also studied by X-ray. They exhibit dimeric, monomeric or polymeric structures, and the N,N-diethylnicotinamide is predominantly coordinated as monodentate pyridine-like N-donor ligand and only a few examples of bridging bonding mode could be found.
The most frequent copper(II) carboxylate complexes with N,N-diethylnicotinamide have general formula [Cu(RCOO)2(dena)2(H2O)2], where RCOO is 2-nitrobenzoate (1) (Figure 1) [3]; 2-chlorobenzoate (2) [4]; 2-bromobenzoate (3) [4]; 4-nitrobenzoate (4) [5]; 2-chloronicotinate (5) [6]; 4-methoxysalicylate (6) [7]; 3-methylsalicylate (7,8) (Figure 2) [7]; 5-methoxysalicylate (9) [7], flufenamate (10) [8]; mefanamate (11) [9]; tolfenamate (12) [10] and 3-methoxysalicylate [11] in [Cu(3-MeOSal)2(dena)2(H2O)2]∙2H2O (13). The coordination environment of the copper atom of all ten compounds is elongated tetragonal bipyramidal, The tetragonal plane of is built up by a pair of unidentate carboxylate anions using carboxylate oxygen atoms [Cu–Oeq in the range 1.96–1.99 Å] and by a pair of neutral N,N-diethylnicotinamide molecules using pyridine ring nitrogen atoms [Cu–Neq in the range1.99–2.04 Å] in trans positions. The axial positions are occupied by the coordinated water molecules [Cu–Owax in the range 2.41–2.52 Å].
Intramolecular hydrogen-bonding interactions involving an axial coordinated water molecule and uncoordinated carboxylate oxygen atom stabilize the molecular structures on all complexes. The molecules of the complexes 1-12 are linked to adjacent molecules through intermolecular hydrogen-bonds between coordinated water molecules and uncoordinated oxygen atoms of N,N-diethylnicotinamide, and they create two-dimensional supramolecular hydrogen-bond networks in iso-structural monoclinic complexes 1-3 , or one-dimensional supramolecular hydrogen-bond chains in two different groups of iso-structural triclinic complexes complexes 4-7 and 8-9. The two groups of triclinic complexes present variability of p-p stacking interactions between two pyridine rings of N,N-diethylnicotinamide and possible orientations of supramolecular hydrogen-bond chains. The both polymorphs of complex [Cu(3-MeSal)2(dena)2(H2O)2] (7) and (8) exists in triclinic form and it is example of supramolecular isomerism [12]. The crystal structures of complex [Cu(4-MeOSal)2(dena)2(H2O)2] (6), measured at two temperatures, gave evidence of the distortion isomerism [13] in this complex. The crystal structures of monoclinic complexes 10-12 consist of complex molecules linked to one-dimensional supramolecular chains by intermolecular hydrogen-bonds, but the carboxylate anions are more complicated and crystal structures are different in comparison with triclinic complexes. The crystal structure of monoclinic complex [Cu(3-MeOSal)2(dena)2(H2O)2]∙2H2O (13) consists of complex molecules as well as uncoordinated water molecules, linked to enriched hydrogen-bond networks.
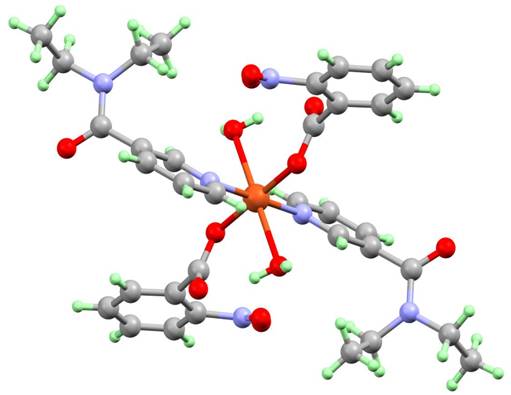
Figure 1. Molecular structure of [Cu(2-NO2Bz)2(dena)2(H2O)2] (1)
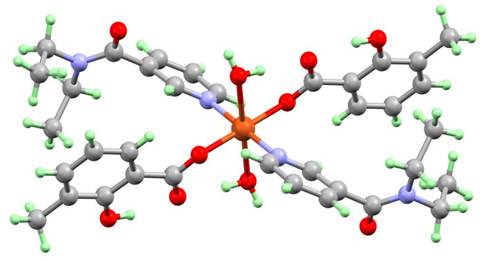
(7)
(8)
Figure 2. Molecular structures of two polymorphs of [Cu(3-MeSal)2(dena)2(H2O)2] (7) and (8).
Acknowledgements.
We thank
References
1. L.B. Zavodnik, V.M. Tsyrkumov, M.I. Bushma,
P.I. Lukienko & L.F. Legonkova, Farmakol.
Toksikol., 54, (1991), 69.
2. F.A. Allen, Acta Cryst., B58,
(2002), 380.
3. J. Kavalírová, M. Korabik, P. Stachová, J.
Moncol, R. Sillanpää, T. Lis, D. Mikloš, M. Melník, J. Mroziński & D.
Valigura, Polyhedron, 27, (2008), 1333.
4. J. Kavalírová, D. Valigura & J. Moncol, Insights into Coordination, Bioinorganic and Applied Inorganic Chemistry, Ed. M. Melník, P. Segľa, M. Tatarko, Press of Slovak University of Technology, Bratislava, 2009, p. 133.
5. T. Hökelek, K. Budak, K. Sendil & H.
Necefoglu, Acta Cryst., C54, (1997), 347.
6. J. Moncol, M. Palicová, P. Segľa, M. Koman,
M. Melník, M. Valko & T. Głowiak, Polyhedron., 21, (2002), 365.
7. Z. Repická, J. Moncol, L. Krupková, D. Hudecová, M. Korabik & D. Valigura, Insights into Coordination, Bioinorganic and Applied Inorganic Chemistry, Ed. M. Melník, P. Segľa & M. Tatarko, Press of Slovak University of Technology, Bratislava, 2009, p. 287.
8. M. Melník,
9. M. Melník, M. Koman, Ľ. Macášková & T.
Głowiak, J. Coord. Chem., 44, (1998), 163.
10. Š. Lörinc, J. Svorec, J. Moncol, M. Melník
& M. Koman, Transition Met. Chem.,
to submitted.
11. J. Moncol, Z. Pučeková, T. Lis & D.
Valigura, Acta Cryst., E62, (2006), m448.
12. B. Moulton & M.J. Zaworotko, Chem. Rev., 101,
(2001), 1629.
13. J. Gažo, Pure Appl. Chem., 38, (1974), 279.