Thermal stability of titanate nanotubes
D. Králová1, R. Kužel2, J. Kováøová1, J. Dybal1, M. Šlouf1
1
Institute of Macromolecular Chemistry,
2
Department of Condensed Matter Physics, Faculty of Mathematics and Physics,
kralova@imc.cas.cz
Keywords: titanium dioxide, titanate nanotubes, electron diffraction.
Abstract
Titanate nanotubes (Ti-NT) were prepared by
hydrothermal synthesis from four different TiO2 powders: anatase
micropowder (mA), rutile micropowder (mR), anatase nanopowder (nA), and rutile
nanopowder (nR). As we use the nanotubes as filler in molten polymers, we
investigated their structural changes at elevated temperatures (up to
Introduction
In the past decade, titanate nanotubes (Ti-NT)
have attracted much attention because of their interesting structure, morphology
and potential applications. A few studies have reported also their thermal
stability at high temperatures. Basically, all were in agreement that structure
of Ti-NT was stable until about
Experimental
Titanate nanotubes (Ti-NT) were synthesized by hydrothermal synthesis as reported in our previous work [6]. Briefly, Ti-NT were synthesized from four different TiO2 powders (anatase micropowder (mA), rutile micropowder (mR), rutile nanopowder (nR), and anatase nanopowder (nA)). Initial concentration of TiO2 was 0.1 g; reaction time was 48 hours.
The morphology of the nanotubes was
investigated by TEM. A droplet of the Ti-NT aqueous suspension was deposited on
a carbon-coated copper grid, left to evaporate and then inspected in a
transmission electron microscope (TEM; Tecnai G2 Spirit 120, FEI,
The crystalline structure and its thermal
stability was also characterized by powder x-ray diffraction (PXRD, diffractograms
at low scattering angles q < 1.4 Å-1, temperatures up to
Results
TEM micrographs (Fig.1a, c) proved that
Ti-NT morphology was not affected by heating up to
Conclusion
The morphology of our laboratory-synthesized
titanate nanotubes was stable at elevated temperatures (up to
References
1. E. Morgado Jr., M. A.S. de Abreu, O. R.C. Pravia, B. A. Marinkovic, P. M. Jardim, F. C. Rizzo, A. S. Araújo, Solid State Sciences, 8, (2006), 888.
2. L.-Q. Weng, S.-H. Song, S. Hodgson, A. Baker, J. Yu, Journal of the European Ceramic Society, 26, (2006), 1405.
3. Y. Lan, X. Gao, H. Zhu, Z. Zheng, T. Yan, F. Wu, S. P. Ringer, D. Song, Adv. Funct. Mater., 15, (2005), 1310.
4. W. Wang, O. K. Varghese, M. Paulose, and C. A. Grimes, Q. Wang and E. C. Dickey, J. Mater. Res., 19, no. 2, (2004)
5. Y.-F. Chena, C.-Y. Lee, M.-Y. Yeng, H.-T. Chiu, Materials Chemistry and Physics, 81, (2003), 39.
6. D. Králová, M. Šlouf, R. Kužel, Materials Structure, 15, no. 2a, (2008), k60.
7. B. D. Yao, Y. F. Chan, X. Y. Zhang, W. F. Zhang, Z. Y. Yang, and N. Wang, Appl. Phys. Lett., 82, No. 2, (2003), 281.
Acknowledgements.
Financial support through grants KAN200520704 a GACR 203/07/0717 is gratefully acknowledged.
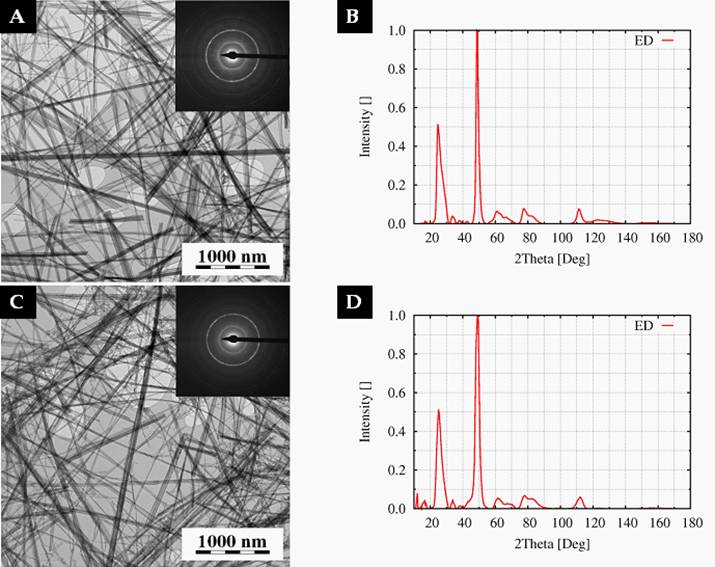
Figure 1. TEM
micrographs and ED patterns of Ti-NT heated up to
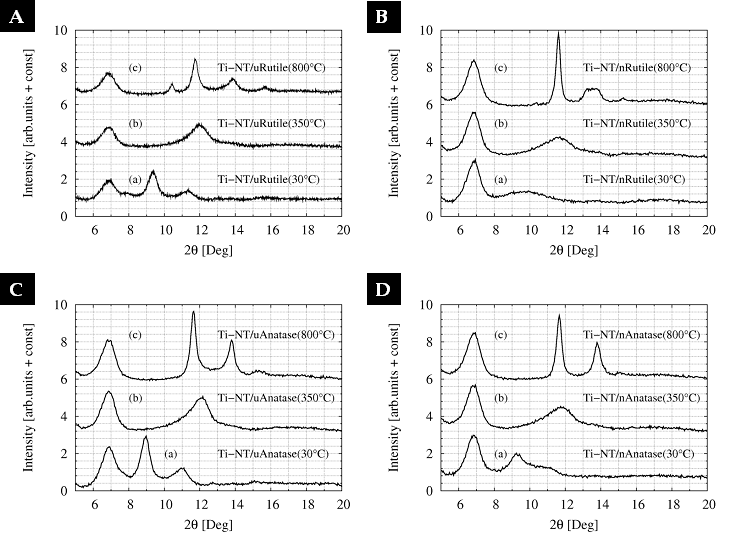
Figure 2. PXRD of Ti-NT synthesized from micro-rutile (A), nano-rutile (B), micro-anatase (C) and nano-anatase showed significant changes.