STRUCTURE OF VALINOMYCIN AND ITS COMPLEXES
Jindřich Hašek 1*, Emanuel
Makrlík2, Michal Dušek3, Ivana Císařová 4,
Jan Dohnálek1, Jarmila Dušková1,
Tereza Skálová1
1 Institute of Macromolecular
Chemistry, AS CR, Heyrovského nám.2, 162 06 Praha 6,
2 Faculty of Applied Sciences, University of West Bohemia, Husova 11, 30614 Plzeň,,
3 Physical Institute,
Academy of Sciences of CR, Cukrovarnická 2, 16000 Praha
6,
4 Institute of inorganic
chemistry, Charles University, Hlavova 2030, 128 40
Praha 2.
hasek@imc.cas.cz
Introduction
Valinomycin is a cyclic dodecadepsipeptide
composed of twelve a-aminoacid-like residues, all with hydrophobic
side chains. More accurately, it can be described as a trimer Ì® [Val – ODVal –DVal - OAla]3
ƒ, where Val is for L-valine, ODVal
for deamino-oxy-D-valine, DVal
for D-valine and OAla for deamino-oxy-L-alanine.
The chemical composition of valinomycin gives it a principle importance in any
living organisms because of its role in selective transport of ions across the
cell membranes. The Cambridge structure database of organic and organometalic
compounds [1] contains 20 records, 16 of them representing independent
observations. All structures deposited in the CCDC except a single one have very
low accuracy of structure determination (R-factors are in the interval 9 % – 19
%). The only exception is a complex of valinomycin with a single water molecule
and 1,5-dioxan determined by Lang in 1992 ( R=3.8 %). Surprisingly,
none of the structures solved by now was with higher water content. Therefore,
we determined two structures with different numbers of water molecules per
single molecule of valinomycin.
Newly determined structures
In the new structures, a single valinomycin molecule is complexed with two waters (R = 4.2 %) or with 12 water
molecules (R = 8.5 %). The results show that the macrocycle
of a single valinomycin folds into a shape similar to the seam on a tennis ball.
In this way, it forms a large barrel with hydrophobic external surface (formed
by side chains of all monomers) and a large hydrophilic cavity inside the
barrel with 12 carbonyls and 6 ether oxygens. In case
that hydrophilic cavity has small volume, the cavity is more spherical. In the case
of higher content of water, the cavity swells and takes an elongated ellipsoidal
shape.
The solved structures show clearly that water molecules concentrate
inside the valinomycin cavity. In excess of water, two valinomycins
form a dimer of the elongated barrel shape filled by
at least 24 water molecules. The external surface of the barrel formed by all
side chains of both valinomycin molecules is highly hydrophobic and therefore
the complexes stack side by side to form layers similar to a membrane. In other
words, the whole system tends to form bilayers of
valinomycin dimers. It is in agreement with the fact
that the measured single crystal with higher water content is formed by
parallel stacking of these bilayers. Figure 1 shows a
perpendicular view on a single layer of the above mentioned bilayer.
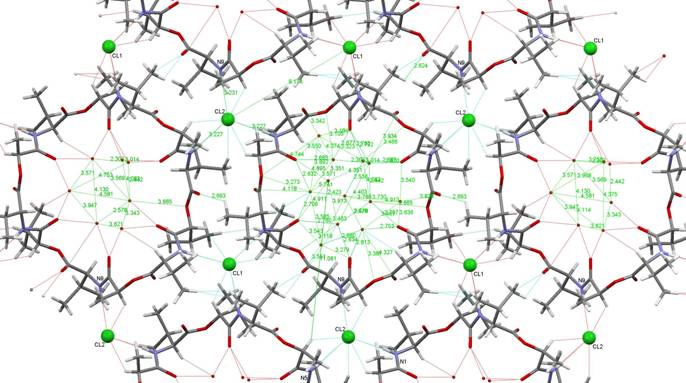
.
Figure 1. A single layer of valinomycin
molecules in the valinomycin-HCl-water
complex with stoichiometry 1:1:12. In the central
valinomycin ring, all water molecules in the tunnel are shown. In the side
rings, only the water molecules directly bound to the valinomycin molecule are present.
Stability of the structure is strengthened by chloride ions (small balls) fixed
in all cases to three amine groups of three neighbor valinomycin molecules.
Valinomycin in the lipid membrane
The pattern formed in the high-water-content structure is an excellent
model for valinomycin action in the cell membrane. Some of single valinomycin
molecules sit on the surface of cell membrane forming thus small cavities and decreasing
locally the thickness of membrane. Other valinomycin molecules form dimers inserted inside the membrane similarly to those
described in the crystal structure. The valinomycin dimers
(barrels with hydrophobic external surface and filled by solution) form water-filled
tunnels across the membrane allowing the passive transport of ions or small
molecules with good affinity for the cavity interior offering 24 carbonyls and
12 ether oxygens for hydrogen bonding on its surface.
These observations explain the mechanism of the valinomycin activity in the
selective transport of ions and small hydrophilic molecules across the cell
membrane. The study brings better understanding of the processes taking place
in living organisms.
Acknowledgement
The
work was supported by projects GA ČR 305/07/1073 and GA AV ČR IAA500500701.
References
1. F.H. Allen, Acta
Crystallogr.,
B58, (2002), 380-388.