Photoionization
for benchmark studies in transition-metal catalysis
Detlef Schröder1
1Institute of Organic Chemistry and
Biochemistry, Czech Academy of Sciences, Flemingovo nám. 2, 16610 Prague 6,
Czech Republic
Keywords: catalysis, photoionization, synchrotron
radiation, vanadium oxide
Abstract
The
concepts for employing photoionization studies with synchrotron radiation for
benchmark studies in transition-metal chemistry are outlined briefly. As an
illustration, the exemplarily case of trimethoxo-vanadium oxide is presented,
where photoionization data essentially helped to establish the entire
thermochemistry of OV(OCH3)3 from the neutral compound to
the quasi-terminal fragments VO+ and VOH+ in the gaseous
phase.
1. Introduction
Experiments
with VUV photons (7 - 60 eV) stemming from synchrotron sources are of outmost
importance as a linkage between modern and advanced experimentation in
chemistry and physics on the one hand and the more and more improving
theoretical tools on the basis of quantum mechanics. Nowadays, one may in fact
state that ab initio theoretical studies of a problem in main-group chemistry
may be more adequate, more accurate, require less personal and infrastructure
and are faster and cheaper than conventional experimentation.
Despite the
enormous progress of quantum theory within the last two decades, these methods
need testing and benchmarking for keeping standards as well as to warrant a
continuous improvement. Moreover, the high standards of accuracy have meanwhile
only been reached for main-group elements, whereas transition-metal compounds
form a considerably more challenging task.
This is the
point of linkage at which experiments with well-resolved VUV photons from a
synchrotron source provide a junction between experiment and theory by means of
the highly accurate determination of atomic or molecular quantities (such as
ionization energies, vibrational levels, excited states etc.) or - in fortunate
cases - even allow the determination of activation barriers of chemical reactions.
While in main-group chemistry, such experiments thus present a test for
existing theoretical tools, in transition-metal research the benchmarks derived
from synchrotron experiments essentially stimulate the progress in the
development of new methods.
2. Methods
for the delivery of benchmark data
Instead of
detailed descriptions of the beamlines or the experimental end-stations used at
the synchrotron facilities, only the general concepts for the establishment of
benchmark data will be introduced. With the availability of tuneable VUV
photons from a synchrotron source, various chemical compounds can be excited
and/or ionized. If samples are used which are sufficiently volatile in
ultra-high vacuum at ambient temperatures (typically up to a few hundreds °C),
photoionization by synchrotron radiation can be combined with mass
spectrometric techniques, which ensures a highly sensitive detection on a
single-event counting basis. As an example, consider a molecular species M
having an ionization energy of 10.0 eV. Below the ionization threshold, the few
M+ cations being formed can be attributed to impact from cosmic
irradiation inside the apparatus (typical count rate 0.03 - 0.1 s-1).
Slightly below the ionization threshold, Rydberg states of the neutral
molecule, M*, can be formed which may eventually autoionize to the molecular
ion M+, but the cross section of these processes is usually very
low. If the photon energy reaches the very ionization threshold, however, the M+
signal increases very rapidly to a plateau regime with typically several 104
counts per second. When the analyzer of the mass spectrometer is fixed on the
mass-to-charge ratio of the molecular ion M+, the photoionization
threshold of M can thus be determined by monitoring M+ as a function
of the wavelength (and the flux) of the ionizing photons. The typical precision
amounts to about ± 0.005 eV for atomic and ± 0.03 eV for molecular species [1];
double photoionization to dications has less favourable threshold
characteristics and thus at best ± 0.1 eV [2,3]. Thresholds for dissociative
ionization are broadened by Franck-Condon effects and thus also only precise by
± 0.1 eV at best [1].
3. Case
study: Ion thermochemistry of trimethoxovanadium oxide OV(OCH3)3
Vanadium-oxide
catalysts play a very important role in a number of large-scale processes such
as in the oxidation of methanol to formaldehyde, the oxidative dehydrogenation
of ethylbenzene, or the industrial production of maleic anhydride. A key
problem in these processes, both partical oxidations, is the minimisation of
competing combustion processes eventually leading to COx. In this respect, the knowledge of the elementary
steps of such oxidation reactions is of prime importance as it can help to
increase the yields of the desired products, thereby reducing the amounts of
byproducts, waste, and heat production.
One way to
achieve detailed insight into the elementary steps of catalytic processes are
gas-phase studies of small model systems, both by experiment and modern quantum
theory. These extensive efforts, on the experimental as well as the
computational sides, need some dedicated benchmarks for evaluation of the
performance of the different methods. In this respect, photoionization
experiments with synchrotron radiation can provide essential information which
cannot be achieved by any other means. As a example, we refer to the
trimethoxovanadium oxide, OV(OCH3)3, which can be
regarded as a model system for C−H bond activations by high-valent
transition-metal oxides [4].
Figure 1
shows the photoion yields of the molecular ion OV(OCH3)3+
(Figure 1a) and the primary fragment HOV(OCH3)2+
(Figure 1b), where the latter is accompanied with the loss of formaldehyde and
hence represents an example of an oxidation reaction. Analysis of the photoion
yields reveals thresholds of (9.56 ± 0.04) eV for the photoionization of the
neutral compound to the cation and (10.1 ± 0.1) eV for dissociative
photoionization to HOV(OCH3)2+ [5]. Both
values provide accurate benchmarks for the calibration of theoretical methods,
in that the former describes the energy demand for removal of one electron from
the vanadium (V) compound, thus resembling defect formation in the solid state,
and the latter turns out to be not due a thermochemical limit imposed by the
exit channel but rather represents the height of the activation barrier for
C−H bond activation.
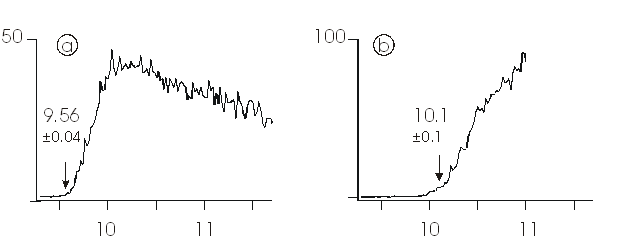
Figure 1. Photoionization yields of (a) the
molecular ion OV(OCH3)3+ and (b) the fragment
ion HOV(OCH3)2+ as a function of the energy of
the photons used to ionise neutral, gaseous HOV(OCH3)2+
[5].
The
usefulness of the synchrotron data is demonstrated by the success of subsequent
work [6], in which starting from neutral OV(OCH3)3 the combined
expertise of experiment and theory could be used to establish the
thermochemistry of trimethoxovanadium oxide from the bulk, neutral compound to
the quasi-terminal fragments VO+ and VOH+, respectively
(Figure 2).
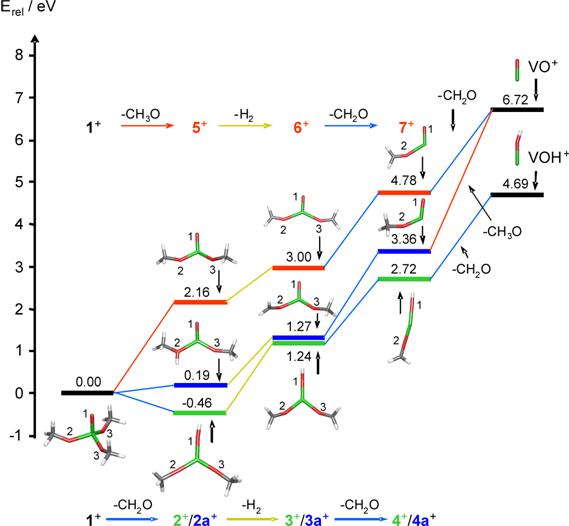
Figure 2. Ion thermochemistry from the
molecular ion OV(OCH3)3+ to the quasi-terminal
fragments VO+ and VOH+.
4. Conclusions
The above
example as well as related work [7,8] demonstrate that photoionization
experiments using synchrotron radiation provide accurate reference data for the
reliable testing and calibration of other experimental methods and for the
critical evaluation of modern theoretical approaches. In this respect, the
exploitation of synchrotron radiation for essays in transition-metal chemistry
is just in its infancy and thus likely to essentially contribute to the future
success in this field.
References
1. D.
Schröder, J. Loos, H. Schwarz, R. Thissen, O. Dutuit, J. Phys. Chem. A 108,
(2004), 9931.
2. J. Roithová, D. Schröder, J. Loos, H.
Schwarz, H.-C. Jankowiak, R. Berger, R. Thissen, O. Dutuit, J. Chem. Phys. 122, (2005), 094306.
3. J. Roithová, J. Žabka, D. Ascenzi, P.
Franceschi, C.L. Ricketts, D. Schröder, Chem.
Phys. Lett. 423, (2006), 254.
4. M. Engeser, D.
Schröder, H. Schwarz, Chem. Eur. J. 11, (2005), 5975.
5. D.
Schröder, J. Loos, M. Engeser, H. Schwarz, C. Jankowiak, R. Berger, R. Thissen,
O. Dutuit, J. Döbler, J. Sauer, Inorg.
Chem. 43, (2004), 1976.
6. D. Schröder, M. Engeser, H. Schwarz, E. C.
E. Rosenthal, J. Döbler, J. Sauer, Inorg.
Chem. 45, (2006), 6235.
7. D.
Schröder, J. Loos, H. Schwarz, R. Thissen, O. Dutuit, Inorg. Chem. 40,
(2001), 3161.
8. Á.
Révész, Cs. I. Pongor, A. Bodi, B.
Sztáray, T. Baer, Organometallics, 25, (2006), 6061.
Acknowledgements.
This work
was supported by the Czech Academy of Sciences (Z40550506).