Phase analysis of multiple absorbed and desorbed Zr-Fe-V by hydrogen
P. Roupcovį, O. Schneeweiss,
1Institute
of Physics of Material, Czech Academy of Science, v.v.i., Brno,
roupcova@ipm.cz
The commercial non-evaporable getter SAES St 707 with chemical composition (70 % Zr, 24.6 % V, and 5.4 % Fe) is using for protection vacuum systems sensitive to presence of hydrogen. We have investigated its phase stability during recharging by hydrogen with emphasis on the influence of impurity formed by residual gases in atmosphere (O2, CO2, H2O).
The surface composition of the as-received
getter exposed by surrounding atmosphere determined by XPS reported in [1-2] consists
of the respective oxides of the getter compounds, i.e., ZrO2, VO2,
and Fe2O3. The getter activated at
We have investigated structure and phase composition of the getter using X-ray powder diffraction (XRD) and Mössbauer spectroscopy (MS). XRD was performed using CoKα radiation with qualitative analysis carried out by HighScore software and the JCPDS PDF-2 database. For a quantitative analysis of the XRD patterns we took HighScore plus with Rietweld structural models based on the ICSD database. 57Fe Mössbauer spectra were measured in a standard transmission geometry using 57Co/Rh source. Isomer shifts δ were refereed relative to α-Fe at room temperature. The computer processing of the spectra done by CONFIT package [3] yielded intensities (atomic fraction of Fe atoms) I of the components, their hyperfine inductions Bhf, isomer shifts δ, quadrupole splittings Δ, and quadrupole shifts εS.
|
|
|
|
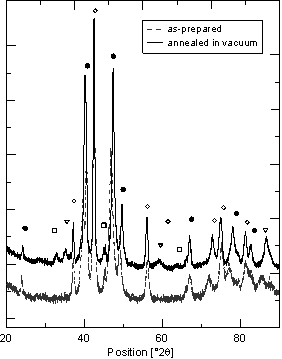
Figure 1. Getter – hydrogen uncharged state. (· cubic C15 and à hexagonal C14 Laves phases, ÿ monoclinic-ZrO2, Ñ cubic- ZrO2). |
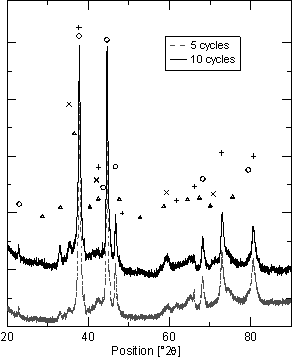
Figure 2. Getter - hydrogen charged state. (+ ZrH2, o ZrV2H3.6, ´ cubic-ZrO2, D monoclinic-ZrO2). |
The getter was hydrided during annealing in
H2 (5N) at
References
1. K. Ichimura, K. Ashida, K. Watanabe, J. Vac. Sci. Technol. A 3 (1985) 2, 346.
2. I. Vedel, L.
Schlabbach, J. Vac. Sci. Technol. A., 11, (1993) 3, 539.
3. T. ˇįk, in Mössbauer
Spectroscopy in Materials Science, edited by M. Miglierini and D. Petridis (
4. L.
Rodrigo, J.A. Sawicki, J. Nucl. Mater. 265 (1999) 208.
5. M. Hara, R.
Hayakawa, Y. Kaneko, K. Watanabe, J. Alloys. Comp. 352 (2003) 218.
Acknowledgements.
This work was supported by the Czech
Ministry of Education, Youth and Sports (1M6198959201) and