Chemistry in the gas phase using the VUV radiation
J. Roithová1
Keywords: Internal Energy, Ionization Energy, Mass Spectrometry, Synchrotron, VUV Radiation
Introduction
The investigation of model ionic systems in the gas phase brings often deep insight into the intrinsic properties of complex systems. For example, we have shown that as small system as Ag2O+/C2H4 is capable to reproduce all reaction steps in the oxidation of ethylene on a silver catalyst [1]. The small models allow not only detailed investigations of kinetics and reaction mechanisms using mass spectrometry, but also ask for a concerted theoretical study. The isolated systems in the gas phase permit the application of high level quantum chemical calculations and direct correlations between the molecular structure and the investigated properties (e.g. ionization energies or proton affinities) or reactivity. On the other hand, lack of solvation leads often to observation of larger reactivity of the ions than it is expected in the condensed phase, which has to be considered in the translation of the results obtained for a model to a real system. Among the biggest disadvantages of the gas-phase experiments is the fact that the internal energy of the generated ions is often not well defined. The state-of-art method for the ionization is usage of the synchrotron radiation. Photons from synchrotron have very well defined energy and the radiation is moreover smoothly tunable. Therefore the conditions for ionization can be precisely defined and the internal energy of the generated ions can be controlled. The tutorial will be devoted to a demonstration of the power of synchrotron experiments for benchmark studies in chemistry and for the reactivity investigations in dependence of internal energy of ions.
Main
objectives of the tutorial
Ionization energies
The simplest application of the power of synchrotron radiation is the determination of ionization energies. Ionization energies can be determined from the dependence of the ion yield on the photon energy. The precision of the obtained value can be very high (± 10 meV) and the limits are usually not set by the energy resolution of the synchrotron radiation, but rather by the properties of the studied molecules, sensitivity of the detection and by the method used for the evaluation of the collected data. Thus, measurements using synchrotron radiation provide us with highly accurate energies, which relates thermodynamic of the charged systems with neutral precursors.
s+(
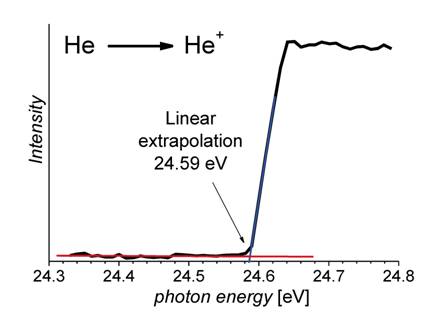
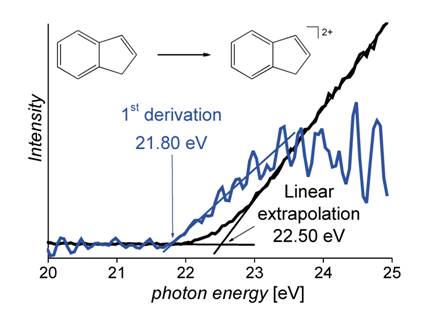
The dependence of the ion yield on the energy of the ionizing photons is described by so-called Wannier law (Equation (1), s+ is cross section and E0 is ionization energy) [2, 3]. For the cross section of singly charged ions is m equal to zero, which means that the onset of the yield of the monocations should appear as a step function. Due to an inevitable imprecision of the experiment caused by thermal broadening and Franck – Condon effects, the onset appears not as a pure step function, but rather as a linear increase of the ionic yield. The threshold is therefore determined as a linear extrapolation of the onset to the baseline (Figure 1). For generation of doubly charged cations, m is equal to one, which means that the ionic yield at the onset is a linear function of the excess of photon energy over the ionization energy. This dependence makes determination of the second ionization energy much more difficult, because the ion yield at E0 is zero and a linear extrapolation of the onset leads to large errors (Figure 2). We have proposed to convert the measured ionization curve to its first derivative and consequently evaluate the derived curve in an analogous manner as it is done for single ionization (Figure 2) [4].
|
|
|
|
Figure 2. Yield of monocations generated from helium as a function of photon energy. Linear extrapolation of the ion yield leads to a value of 24.59 ± 0.01 eV. Note that the ion yield shows almost perfect step behavior as it is expected for atomic monocations (measured at ELETTRA). |
Figure 3. Yield of dications generated from indene as a function of photon energy. Linear extrapolation of the ion yield leads to a value of 22.50 ± 0.10 eV, whereas an analogous evaluation of the derived curve leads to a value of 21.80 ± 0.10 eV (measured at ELETTRA). |
Internal energies
The reactivity of ions is drastically influenced by their internal energy. Conventional ionization of neutral molecules can lead to highly vibrationally excited ions or can even yield electronically excited species. The observed reactivity can be thus due to vaguely defined excited states of the reactants. Synchrotron radiation is a unique tool for the preparation of reactant ions with defined internal energy and for the investigation the reactivity in dependence of the internal energy.
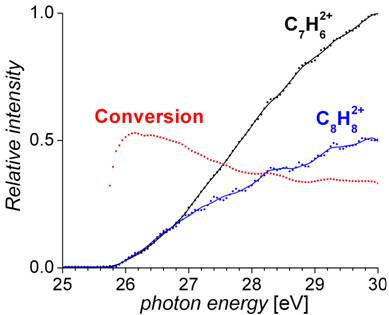
The effect of the internal energy of reactants on their reactivity will be shown on an example of a reaction of the C7H62+ dication with methane. It has been shown that medium sized dications easily undergo a coupling reaction with methane to yield larger hydrocarbons (Equation (2)) [5]. This reaction can be of high relevance for the explanation of the hydrocarbon growth in extreme environments as interstellar medium of planetary atmospheres. To this end, it is particularly important to exclude the possibility that observed reactivity is caused by internally excited ions. The first results from the beamline DESIRS of SOLEIL demonstrate that indeed the internally cold ions react fastest (Figure 3).
CnHm2+ + CH4 → [Cn+1Hm+4]2+
→ Cn+2Hm+22+
+ H2 (2)
|
|
|
Figure 3. Yield of the doubly charged product C8H82+ from the reaction of C7H62+ and CH4 as a function of photon energy used for generation of C7H62+ from toluene. Note that the conversion decreases with increasing internal energy of the C7H62+ reactant (measured at SOLEIL). |
References
1. J. Roithová, D. Schröder, J. Am. Chem. Soc., 129, (2007), 15311.
2. G. H. Wannier, Phys. Rev., 90 (1953), 817.
3. G. H. Wannier, Phys. Rev., 100 (1955), 1180.
4. J.
Roithová, J. Žabka, D. Ascenzi, P.
Franceschi, L. Ricketts, D. Schröder, Chem. Phys.
Lett., 423, (2006), 254.
5. C. L. Ricketts, D. Schröder, Ch. Alcaraz, J. Roithová, Chem. Eur. J., 14, (2008), 4779.