Crystallographic
study of pyrite related phases: PtSnS, PtSnSe and PtSnTe
F. Laufek1, J. Plášil2
1Czech Geological Survey, Geologická 6, 152 00, Praha 5
2Charles University, Faculty of Science, Albertov 6, 128 43 Praha 2
frantisek.laufek@geology.cz
This
presentation is a continuation of our systematic investigations on crystal
structures and selected physical properties of M-X-Ch compounds of nickel-group
metals (M = Ni, Pd, Pt) and main group IV. and VI. elements (IV = Si, Ge, Sn; VI = S, Se, Te). These phases are
of interest in materials science because of their possible thermoelectric
applications. Moreover, as was mentioned by [1], many of these compounds show
interesting structural features of the pyrite (FeS2, Pa3)
type structural family. This is because the presence of X-X or X-Ch pairs and
related ordering phenomena [1].
The ternary
compounds PtSnS, PtSnSe and
PtSnTe were synthesised from elements by conventional
high temperature solid state reactions. Stoichiometric amounts of Pt (99.9%),
Sn (99.99%), S (99.995%), Se (99.99%) and Te (99.99%) were sealed in evacuated
silica tubes and heated for 800 °C for 1 day. Following this, the samples were
ground using agate mortar and pestle, and sealed again and heated at 800 °C for
one week. The resultant material was once again ground and heated at 800 °C for
two weeks. Finally, the samples were quenched in cold water.
The
existence of PtSnS, PtSnSe
and PtSnTe compounds is given in [1]. Also a
relationship of these phases to the pyrite structural family (more specifically
to the cobaltite type) is proposed [1]. However, no structural details
including atomic coordinates are given. Here we report a detailed structural
study of title phases. As single crystals of sufficient quality were not
available, the structural analyses were performed on powder samples. The
structures of title compounds can be derived from the pyrite structure (FeS2)
replacing of S-S dumbbells by X-Ch anion pair. For similar structures three
arrangements of the ordering of anionic atoms were proposed [1, 2]. One
possibility corresponds to the ullmanite type
structure (NiSbS, P213), which
retains cubic symmetry. Another option of ordering of anionic atoms represents
the cobaltite type structure (CoAsS, Pca21).
Also an intermediate possible structure model was described in space group R3
[1]. To determine which ordering scheme can be applied for PtSnS,
PtSnSe and PtSnTe careful
analysis of powder diffraction patterns was done. The powder
diffraction patterns of title compounds and pyrite are very similar. However,
the presence of additional diffractions indicating the ordering with respect to
lowering symmetry and splitting of specific diffractions demonstrating
deviations from cubic lattice, revealed the CoAsS
structure model for PtSnS, PtSnSe
and PtSnTe. Final refinement was done by Rietveld
method using FullProf program [3].
PtSnS, PtSnSe and PtSnTe display
orthorhombic symmetry, space group Pca21. In these three
compounds, Pt is surrounded by three Sn and X atoms showing distorted
octahedral coordination. These [PtSn3X3]
octahedra are connected by corner-sharing. An important feature presents in the structure of
title compounds is the existence of Sn-X pairs (Figure 1).
1. R.
Weihrich, D. Kurowski, A.C. Stűckl, S. Matar, F. Rau, T. Bernert, J. Solid State Chem., 177,
(2004), 2591.
2. A.J. Foecker, W. Jeitschko, J. Solid State Chem., 169, (2001), 69.
3. J. Rodríguez-Carvajal,
FullProf.2k, Laboratoire Léon Brillouin, France, 2006.
This study was supported by the Grant Agency of the Academy of Sciences of the Czech Republic (Project No. KJB 300130612) and by the internal project of the Czech Geological Survey (Project No. 323000).
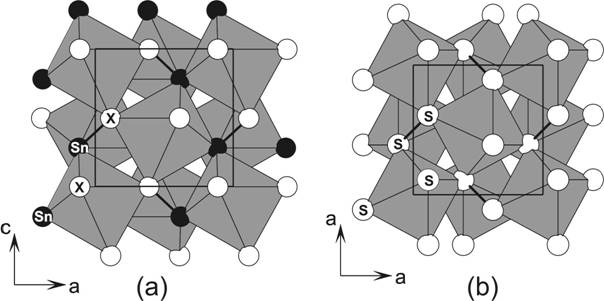
Figure 1. (a) Polyhedral representation of
PtSnX (X = S, Se, Te) structures (space group Pca21)
showing the [PtSn3X3] octahedra and Sn-X
pairs. (b) Structure of pyrite (FeS2, space
group Pa3) is shown for comparison.