Synthesis of titanate nanotubes: influence
of TiO2 modification on crystalline structure and morphology
D. Králová
1Institute
of Macromolecular Chemistry, Academy of Sciences of the Czech Republic,
Heyrovskeho nam. 2, 162 06 Praha 6,
2Member of Consortium for Research of Nanostructured and Crosslinked Polymeric Materials (CRNCPM)
3Department of Electronic Structures, Faculty of Mathematics and Physics, Charles University,
121 16 Praha 2, Ke Karlovu 5, Czech Republic
kralova@imc.cas.cz
Keywords: TiO2 microparticles and
nanoparticles, titanate nanotubes, electron diffraction.
Abstract. This work is focused on preparation of titanate nanotubes (Ti-NT) from various modifications of TiO2. Different source micro/nanopowders of TiO2 yielded Ti‑NT with similar sizes, shapes and structures. Residual TiO2 particles in final Ti-NT suspensions were eliminated by modification of preparation procedure. Morphology and crystalline structure of both source TiO2 and final Ti-NT were investigated by means of electron microscopy, electron diffraction and X-ray diffraction.
Introduction
Recently discovered titanate nanotubes [1] attracted special attention in the nanomaterial research due to their novel unique crystalline structure and morphology [2-4] as well as the mechanism of their formation [5-7]. The nanoparticles are basically rolled sheets of titanate. Their outer diameter is about 10 nm and their length varies from 100 nm to 1 μm. Nanotubes are obtained by simple hydrothermal process without using of templates. Due to their high aspect ratio, titanate nanotubes (Ti-NT) have potential use as nanofillers in polymer composites, similarly to carbon nanotubes. They may not exhibit as unique properties as carbon nanotubes, but they are highly uniform, their fabrication is simple, inexpensive and highly reproducible.
In our previous work we developed an isolation method yielding non-destructed and non-merged nanotubes from aqueous solution in gram-scale amounts [8]. Problem of our isolation method consisted in that we had always obtained a mixture of Ti-NT and anatase or rutile as proved by powder X-ray diffraction. Main objective of this work was to refine the synthesis so that we get rid of residual TiO2. Moreover, we wanted to compare the differences among syntheses starting from various TiO2 crystal sizes and modifications. The morphology of both source TiO2 and final Ti-NT was investigated by means of scanning and transmission electron microscopy. The crystalline structure was studied by X-ray and electron diffraction.
Experimental
Synthesis of titanate
nanotubes. Starting TiO2
modifications included: technical powder (Riedel-de Haën, Sigma-Aldrich),
anatase TiO2 nanopowder (99,8%; Aldrich), rutile micropowder (99,9%,
Aldrich) or rutile nanopowder (99,9%, Aldrich). In the following text, the TiO2
powders are denoted as follows: technical powder - microanatase (mA),
anataseTiO2 nanopowder – nanoanatase (nA), rutile micropowder –
microrutile (mR), rutile nanopowder – nanorutile (nR). Titanate nanotubes (Ti-NT)
were synthesized by hydrothermal synthesis as reported in our previous work [8], but several modifications were made. Firstly,
three different concentrations of TiO2 technical powder were used:
Scanning electron microscopy. All Ti-NT (or TiO2) aqueous suspensions were sonicated 1 min just before final preparation for electron microscopy. A dropl of Ti-NT/TiO2 suspension was deposited on bulk carbon support and left to evaporate. After complete evaporation, the carbon support with investigated particles was transferred into scanning electron microscope (SEM) Quanta 200 FEG (FEI, Czech Republic). The specimens were observed as they were in low-vacuum mode using accelerating voltage 30 kV.
Transmission electron microscopy and electron diffraction. The specimens were sonicated as described in the previous paragraph. A droplet of the sonicated suspension was deposited on a thin, transparent carbon film, left to evaporate and then inspected in a transmission electron microscope (TEM; Tecnai G2 Spirit 120, FEI, Czech Republic). The specimens were studied in both bright field (conventional TEM/BF) and selected-area electron diffraction (TEM/ED) modes at 120 kV.
Powder X-ray diffraction. Powder X-ray diffraction (PXRD) measurements of whole patterns were performed mainly on XRD7 (FPM-Seifert) diffractometer with monochromator in the diffracted beam. The PXRD results were compared with simulated powder diffraction patterns (calculated by program PowderCell, [9]) and experimental ED diffraction patterns (processed by program ProcessDiffraction, [10]).
Results and discussion
Hydrothermal synthesis of titanate nanotubes can be schematically described by very simple reaction: TiO2 ® Ti-NT, which usually takes place at elevated temperatures > 100 oC and high concentrations of NaOH. In this study, the first variable parameter of the syntheses was the initial concentration of source TiO2 powders. The next two variables were average particle size and crystalline modification of source TiO2 powders. The size of TiO2 particles was characterized by SEM as shown in Fig. 1. Size of particles was very different (mA: 500nm - 100nm; mR: 1µm - 300nm; nA: 300nm - 20nm; nR: 90nm - 20nm). Absolute purity of anatase (Fig. 1a,c) and rutile (Fig. 1b,d) modifications was proved by both electron and X-ray diffraction.
PXRD patterns in figure
2 illustrate influence of initial concentration TiO2 on purity of
synthesized Ti-NT. The reaction time was prolonged from 20 h (ref. [8]) to 48 h
(this work). If
TEM/BF micrographs and TEM/ED patterns in Fig. 3 show influence of TiO2 crystallite size and modification on Ti-NT morphology and crystalline structure. TEM results proved that all crystal modifications and sizes of source TiO2 (Fig. 1) can be used for synthesis of Ti-NT (Fig. 3, BF micrographs). Closer inspection of both TEM and SEM micrographs suggested that the finest and thinnest nanotubes were synthesized from powder of nanorutile, nR.
Electron diffraction (Fig. 3 and Fig. 4) re-confirmed that all single nanotubes of Ti-NT had the same structure, with three characteristic diffractions (2theta = 24, 28 and 48deg for CuKapha), which is in agreement with our previous work [8]. However, powder X-ray diffraction suggested that the crystalline structure of Ti-NT prepared from micropowders (Fig. 4a,b) differed from the structure of Ti-NT prepared from nanopowders (Fig. 4c,d). In case of nanopowder-based Ti-NT, the ED and PXRD were quite similar, showing tree characteristic Ti-NT diffractions (at 24, 28 and 48deg). The other intensive peak in PXRD (at 10deg) could not be observed at ED as it was hidden by beamstopper. In case of micropowder-based Ti-NT, the ED and PXRD diffraction patterns were very different. Our tentative explanation is as follows: In case of ED, the diffraction pattern had to be recorded from locations where the specimens were thin enough to be transparent for electrons. In such places the specimens contained single nanotubes and, as a result, all ED diffraction patterns are the same. In case of PXRD, the diffraction patterns were recorded from the whole specimens. Micropowder-based Ti-NT contained higher amount of nanotube agglomerates in comparison with nanopowder-based Ti-NT, which could be observed by SEM. The agglomerates had probably different crystalline structure (cf. Figs 4a-b and 4c-d). Moreover, the agglomerates were too big for ED (non-transparent for electrons) but their diffractions dominated in PXRD (much bigger crystallites). Consequently, the PXRD and ED patterns of micropowder-based Ti-NT were different.
Conclusion
Hydrothermal synthesis
of Ti-NT, described in our previous work [8], was further investigated and
optimized. Variable parameters during Ti-NT syntheses were: (a) initial
concentrations of TiO2, (b) total reaction times, (c) average
particle size of source TiO2 powders and (d) crystalline
modifications of source TiO2 powders. Morphology of Ti-NT was
investigated by both microscopic (SEM, TEM) and diffraction methods (ED, PXRD).
The results could be summarized as follows: (i) Residual TiO2 particles in final Ti-NT suspensions were
eliminated by lower concentration of source TiO2 powders (
References:
[1] Kasuga T. Hiramatsu M., Hodin A., Sekino T., Niihara K., Formation of Titanium Oxide Nanotube, Langmuir 1998; 14: 3160-3163
[2] Morgado Jr. E., de Abreu M. A. S., Pravia O. R. C., Marinkovic B. A., Jardim P. M., Rizzo F. C., Araújo A. S., A study on the structure and thermal stability of titanate nanotubes as a function of sodium content, Solid State Sciences 2006; 8: 888–900
[3] Tsai C.-C., Teng H., Structural Features of Nanotubes Synthesized from NaOH Treatment on TiO2 with Different Post-Treatments, Chem. Mater. 2006; 18: 367-373
[4] Ma R., Bando Y., Sasaki T., Nanotubes of lepidocrocite titanates, Chemical Physics Letters 2003; 380: 577–582
[5] Yao B.D., Chan Y.F., Zhang X.Y., Zhang W.F., Yang Z.Y., Wang N., Formation mechanism of TiO2 nanotubes, Applied Physics Letters 2003; 82 (2): 281-283
[6] Wang W., Varghese O.K., Paulose M., Grimes C.A., A study on the growth and structure of titania nanotubes, J. Mater. Res. 2004; 19 (2): 417-422
[7] Weia M., Konishia Y., Zhoua H., Sugiharaa H., Arakawa H., Formation of nanotubes TiO2 from layered titanate particles by a soft chemical process, Solid State Communications 2005; 133: 493–497
[8] Králová D., Pavlova E., Šlouf M., Kužel R., Preparation and structure of titanate nanotubes, Materials Structure 2008; 15 (1): 41-45
[9] http://www.ccp14.ac.uk/tutorial/powdcell/index.html
[10] J. L. Lábár, Microscopy and Analysis, 75 (2002) 9-11.
The authors are indebted for financial support
trough grants MSMT 2B06096 (Ministry of Education, Youth and Sports of the
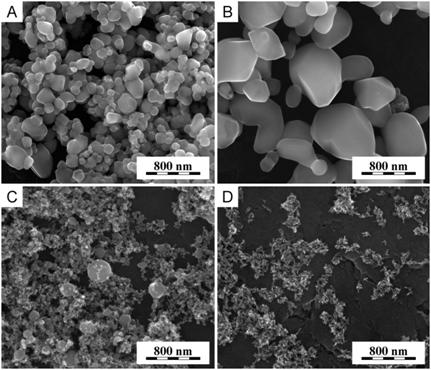
Figure 1. Various TiO2 particles for the syntheses: (a) microparticles of anatase (mA), (b) microparticles of rutile (mR), (c) nanoparticles of anatase (nA) and (d) nanoparticles of rutile (nR).
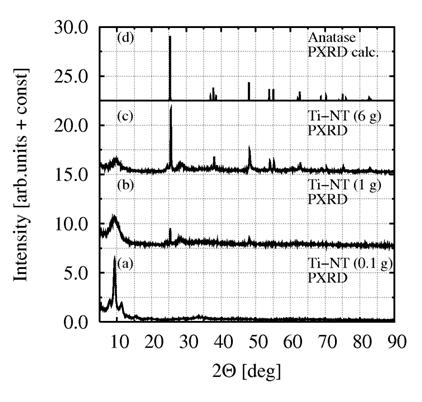
Figure 2. Ti-NT synthesized from different concentration of TiO2: (a)
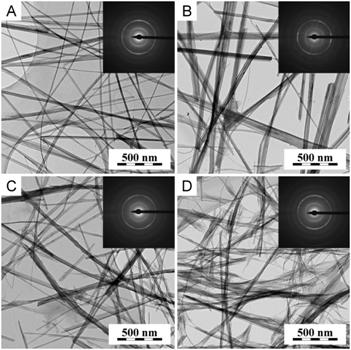
Figure 3. Ti-NT synthesized from: (a) microanatase, (b) microrutile, (c) nanoanatase and (d) nanorutile. TEM micrographs showed different thickness and length of each type of nanotubes, ED patterns did not show any difference.
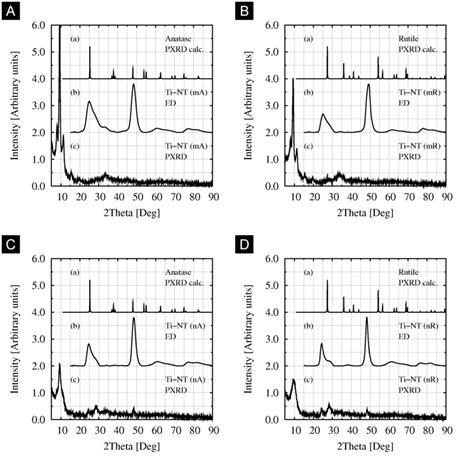
Figure 4. Influence of TiO2 modification on Ti-NT crystalline structure. Comparison of calculated and experimental diffraction patterns of Ti-NT prepared from: (a) microanatase, (b) microrutile, (c) nanoanatase and (d) nanorutile. Single nanotubes always showed the same diffraction patterns (see ED in cases a,b,c,d), but larger agglomerates could be different (cf. PXRD in a,b and c,d).