Painting copper-based pigments, their chemism and degradation
S. Švarcová1,
J. Schweigstillová2,
1Institute
of Inorganic Chemistry AS CR, v.v.i., ALMA laboratory, 250 68 Husinec-Řež,
2Institute
of Rock Structure and Mechanics AS CR, v.v.i., V Holešovičkách 41, 182 09 Praha
8,
The art objects with porous nature, such as wall paintings or sandstone statues, are often exposed to attack of salts present in surrounding environment which results in deterioration of these art works. Crystallizing salts may be locally concentrated as efflorescence on the art work surface or as invisible subflorescence in the subsurface bulk of the porous materials that causes mechanical damages of artworks. Additionally, the presence of salts can also lead to the decay or alteration of pigments accompanied by a change of the original colour [1, 2].
Because of their sensitivity to moisture and air pollution, some pigments were not recommended for using in wall paintings. However, we commonly find some of them in real samples. Salts of copper widely used as relatively cheap blue and green pigments are very good example. Many copper-based pigments are basic salts that are metastable if the activity of other ions changes during wetting the colour layer by salt solution. Conversion to further salt can change the pigment colour, but even this fact cannot be easily revealed by examination of the artwork in its present state. One of the reasons is that it is not always possible to distinguish original phases and the products of their salt attack. Malachite, as well as basic copper chlorides, atacamite and paratacamite, are sometimes supposed to be the degradation products of mineral pigment azurite [3]. On the other hand, malachite, atacamite and paratacamite were reported also as used pigments [4].
We have investigated the composition of real samples as well as we have performed several types of model experiments in order to study the possible interaction between selected pigments and salt solutions. Chosen salts either occur in the environment (e.g. Na2SO4, CaSO4, NaCl, NaNO3, Ca(NO3)2, urea) or their presence in an art work is a result of some restorer action (e.g. Chelatone III, NaHCO3, (NH4Cl )2CO3) In the first type of experiments we mixed selected copper-based pigments, i.e. azurite, malachite and neutral verdigris, with a range of salt solutions and left them to react for ca two months. The reaction products were analyzed using powder XRD. Simultaneously we painted azurite and malachite on the surface of building bricks experimental bodies covered by trilaminar plaster prepared according the historical recipes. We used both historical techniques for painting, “secco” on dry plaster and “genuine fresco” on wet plaster. In the case of “secco” technique we exposed experimental bodies to selected solutions that evaporated through the porous body affecting finally the colour layer. Colour changes were monitored and products of alterations were measured as powder using powder X-ray diffraction in Bragg-Brentano geometry and as fragment of colour layer using powder X-ray micro-diffraction. In the case of “fresco” the both painted surfaces got grey till the following day, thus we did not perform further experiments.
Powder X-ray micro-diffraction could
significantly enhance the detection of minor phases, as demonstrated in Fig.
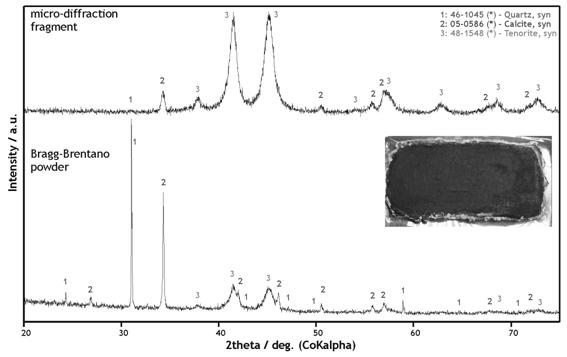
Figure 1. The comparison of diffractograms measured in Bragg-Brentano and microdiffraction geometry on the corroded malachite model layer.
References
1. S.G. Schirripa & D. Paoletti, J. optics-nouvelle revue d optique, 70, (1996), 133.
2. B. Salvadori, V. Errico, M. Mauro, E. Melnik, L. Dei, Spectroscopy letters., 36, (2003), 501.
3. L. Dei, A. Ahle, P. Baglioni, D. Dini, E. Ferroni, Studies in Conservation, 43, (1998a), 80.
4. D. Scott, Studies in Conservation, 45, (2000), 39.
Acknowledgements
The authors appreciate the Grant
Agency of AS CR for the financial support of the project No. B400320602.